1. Background
On 31 December 2019, the China Health Authority warned the World Health Organization of several cases of pneumonia with unknown etiology in China (1). On 07 January 2020, health officials confirmed that the disease is caused by a new virus that had not yet been identified. The causative agent of the disease belonged to the Orthocoronavirinae subfamily in the Coronaviridae family with different behaviors compared to other coronaviruses, such as Middle-East respiratory syndrome coronavirus from 2012 to 2015 and severe acute respiratory syndrome coronavirus in 2003. The disease caused by the virus (known as COVID-19) spread to other parts of China, as well as the world, leading to global epidemics thus far (2-4). The disease is transmitted by inhalation or contact with infected droplets, and its incubation period is 2 - 14 days (five days on average) (5, 6).
The clinical symptoms of COVID-19 vary from asymptomatic to acute respiratory distress syndrome and dysfunction in multiple organs. The disease has no specific symptoms, but the most common ones are fever, cough, dyspnea, sore throat, headache, fatigue, and myalgia. In some cases, extra-pulmonary symptoms, such as loss of the senses of taste and smell, conjunctivitis, and gastrointestinal symptoms, are also reported. Severe complications of the disease include acute pulmonary injury, acute respiratory distress syndrome, shock, and acute renal impairment (7).
Some patients with COVID-19 require hospitalization and need breathing support by ventilator machines and stay at the intensive care units (ICUs). The time from the onset of the symptoms to severe hypoxia and admission to ICU is approximately 7 to 12 days (8). Although some risk factors for severe COVID-19, including age above 65, diabetes, hypertension, cardiovascular disease, chronic respiratory disease, and immunodeficiency (7), are known, there is a lack of evidence to confirm this relationship, and the results of studies are also contradictory. On the other hand, it is confirmed that age is a key factor in many infectious and non-infectious diseases, and clinical signs and risk factors for disease can vary between the elderly and the young (9-11).
2. Objectives
The current study aimed to compare clinical manifestations and risk factors for death in two groups of patients with COVID-19 aged under and above 50 in Qom Province, as the first city in Iran that cases with the coronavirus were identified in it.
3. Methods
The current retrospective cohort study was performed on patients with COVID-19 in Qom Province, Iran. The list of patients with confirmed COVID-19 hospitalized until April 2020 in Qom Province was prepared. Patients were divided into four groups: the first group included patients aged under 50 discharged after recovery; the second group included patients aged under 50 who died from COVID-19; the third group consisted of patients aged above 50 discharged after recovery, and the fourth group included patients above 50 years who died from COVID-19. Patients who had not yet been discharged or not fully recovered during the study period were excluded. Patients aged less than 18 years were not included in the study. A definitive diagnosis of COVID-19 was made based on the positive results of the real-time PCR assay. Briefly, collected nasopharyngeal swabs were placed in the sample collection tubes containing a viral transport medium and were immediately transferred to the coronavirus-reference laboratory. Reverse transcription real-time polymerase chain reaction (RT-PCR) was performed using specific primer/probe sets according to the study done by Corman et al. (12). From each group, 45 patients were randomly selected based on the table of random numbers. One patient from both of the second and third groups was excluded during data analysis from the study because of the incomplete recording of data. Finally, the data of a total of 178 patients were analyzed.
Data of each patient were extracted based on hospital records and interviews with the patient and his/her relatives. The collected information included demographic characteristics (i.e., age, gender, nationality, height, and weight), symptoms on admission (i.e., fever, cough, shortness of breath, headache, diarrhea, nausea and vomiting, loss of senses of taste and smell), and risk factors for disease (i.e, gender, body mass index (BMI), smoking tobacco and hookah, addiction to drugs, diabetes, history of hypertension, chronic respiratory disease, and immunodeficiency). Patients who were treated with immunosuppressive agents (i.e., corticosteroids, chemotherapy, post-transplantation drugs, etc.) or had immunodeficiency caused by disease were classified as immunocompromised. After the collection of the data, the risk factors for death from COVID-19 were compared between the two age groups. Data were analyzed with SPSS software using t-test, chi-square, and logistic regression tests. The research proposal was approved by the Ethics Committee of the Qom University of Medical Sciences (code number: IR.MUQ.REC.1399.001), and principles regarding the confidentiality of participants’ information were observed during the extraction and publication of the data.
4. Results
In the present study, 178 patients with COVID-19 in Qom Province were enrolled. The mean age of the patients was 51.96 ± 16.74 years. The most common symptom in hospitalized patients was dyspnea (82.6%), followed by cough (73.6%) and fever (71.9%). The most prevalent gastrointestinal symptoms were nausea and vomiting (40%), followed by diarrhea (27.5%). Also, loss of senses of smell and taste were reported in 24.2% and 28.1% of the hospitalized patients, respectively. The patients in the two age groups under and above 50 were included in the study (n = 89 in each group). The distribution of patients had no significant differences between the two groups in terms of gender, nationality, and BMI values (Table 1).
| Variable | Age Group | P-Value | ||
|---|---|---|---|---|
| < 50 y/o | > 50 y/o | Total | ||
| Age, mean ± SD | 38.3 ± 7.96 | 65.61 ± 11.16 | 51.96 ± 16.74 | - |
| Sex ratio (male/female) | 60/29 | 62/27 | 122/56 | 0.747 |
| Non-Iranian nationality, N (%) | 12 (13.5) | 9 (10.2) | 21 (11.9) | 0.503 |
| BMI, mean ± SD | 29.24 ± 7.24 | 28.49 ± 5.43 | 28.89 ± 6.4 | 0.441 |
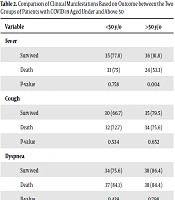
The distribution of clinical symptoms based on patients’ outcomes (death and recovery) in the two age groups is shown in Table 2. In the group aged above 50, the prevalence of fever was higher in the recovered subjects than in dead ones (81.8% vs. 53.3%). In addition, headache and loss of the senses of smell and taste were more common in the recovered subjects in both age groups.
| Variable | <50 y/o | >50 y/o |
|---|---|---|
| Fever | ||
| Survived | 35 (77.8) | 36 (81.8) |
| Death | 33 (75) | 24 (53.3) |
| P-value | 0.758 | 0.004 |
| Cough | ||
| Survived | 30 (66.7) | 35 (79.5) |
| Death | 32 (72.7) | 34 (75.6) |
| P-value | 0.534 | 0.652 |
| Dyspnea | ||
| Survived | 34 (75.6) | 38 (86.4) |
| Death | 37 (84.1) | 38 (84.4) |
| P-value | 0.439 | 0.798 |
| Headache | ||
| Survived | 29 (64.4) | 22 (80) |
| Death | 17 (38.6) | 13 (28.9) |
| P-value | 0.015 | 0.042 |
| Diarrhea | ||
| Survived | 19 (42.2) | 11 (25) |
| Death | 12 (27.3) | 7 (15.6) |
| P-value | 0.139 | 0.267 |
| Nausea and vomiting | ||
| Survived | 24 (53.3) | 19 (43.2) |
| Death | 16 (36.4) | 13 (28.9) |
| P-value | 0.108 | 0.16 |
| Lack of smell | ||
| Survived | 18 (40.9) | 15 (34.1) |
| Death | 7 (15.9) | 3 (6.7) |
| P-value | 0.009 | 0.001 |
| Lack of taste | ||
| Survived | 21 (47.7) | 18 (40.9) |
| Death | 8 (18.2) | 3 (6.7) |
| P-value | 0.003 | < 0.001 |
The distribution of risk factors based on the outcomes (death and recovery) in the two age groups is shown in Table 3. Among the risk factors investigated in the current study, only the distribution of BMI based on the outcome of the disease was significantly different between the two groups, so that in the age group under 50, the subjects who died had higher BMI values, but in the age group above 50, patients who died had lower BMIs.
| Variable | < 50 y/o | > 50 y/o |
|---|---|---|
| Sex ratio (male/female) | ||
| Survived | 30/15 | 30/14 |
| Death | 30/14 | 32/13 |
| P-value | 0.879 | 0.764 |
| BMI | ||
| Survived | 27.71 ± 4.67 | 29.65 ± 6.33 |
| Death | 30.75 ± 8.88 | 27.36 ± 4.15 |
| P-value | 0.049 | 0.049 |
| Smoking, No (%) | ||
| Survived | 7 (15.6) | 7 (15.9 %) |
| Death | 8 (18.2) | 4 (8.9 %) |
| P-value | 0.741 | 0.248 |
| Addiction, No (%) | ||
| Survived | 2 (4.4) | 2 (4.5) |
| Death | 0 | 3 (6.7) |
| P-value | 0.253 | 0.511 |
| Hypertension, No (%) | ||
| Survived | 5 (11.1) | 19 (43.2) |
| Death | 6 (13.6) | 18 (40) |
| P-value | 0.717 | 0.761 |
| Diabetes, No (%) | ||
| Survived | 8 (17.8) | 16 (36.4) |
| Death | 13 (29.5) | 23 (51.1) |
| P-value | 0.191 | 0.161 |
| History of chronic respiratory disease | ||
| Survived | 11 (24.4) | 10 (22.7) |
| Death | 7 (15.9) | 12 (26.7) |
| P-value | 0.316 | 0.667 |
| Immunodeficiency a | ||
| Survived | 4 (8.9) | 3 (6.8) |
| Death | 7 (15.9) | 3 (6.7) |
| P-value | 0.314 | 0.977 |
aDue to drug use or disease
The results of logistic regression based on the outcome of the disease (death and recovery) in both age groups showed that among the variables investigated, BMI value had a significant relation with the disease outcome in both age groups so that higher BMI value was considered the risk factor and preventive factor for the age groups under and above 50, respectively (odds ratios = 1.11 Vs. 0.89). In the age group under 50, immunodeficiency was the risk factor for death; however, the difference between the groups was insignificant. Diabetes was also a risk factor for death in both age groups; however, it was not statistically significant (Table 4). Among the clinical symptoms, loss of sense of taste in both age groups was a protective factor against death; fever was a protective factor against death in the age group above 50. In analysis of total patients, the headache was found as a protective factor against death (OR = 0.49) (Table 5).
| Variable | < 50 y/o | > 50 y/o | Total |
|---|---|---|---|
| BMI, odds ratio (95% CI) | 1.11 (1.011 - 1.22) | 0.888 (0.795 - 0.993) | - |
| Immunodeficiency, odds ratio (95% CI) | 3.179 (0.797 - 12.676) | - | - |
| Diabetes, odds ratio (95% CI) | 3 (0.951 - 9.46) | 2.317 (0.930 - 5.771) | 1.921 (1.007 - 3.664) |
| Variable | < 50 y/o | > 50 y/o | Total |
|---|---|---|---|
| Lack of taste | 0.243 (0.092 - 0.641) | 0.138 (0.036 - 0.536) | 0.211 (0.097 - 0.460) |
| Fever | - | 0.4 (0.144 - 1.11) | - |
| Headache | - | - | 0.495 (0.258 - 0.950) |
5. Discussion
The current study aimed at investigating some of the risk factors for death in patients with COVID-19. The results of the present study showed that male patients were more at risk for hospitalization because of COVID-19, but the mortality rate in hospitalized patients did not vary by gender. Some studies did not show any relationship between gender and disease outcomes (13, 14). Also, some studies show that there might be a sex predisposition to COVID-19. In a study on 191 hospitalized patients in China, 62% of the subjects were male (13). Some researchers have suggested that this difference might be associated with the much higher smoking prevalence in men; also, authors show that in current smokers, ACE2 expression was significantly higher, especially in Asian ethnicity (15).
The most common symptoms in hospitalized patients were dyspnea, cough, and fever, respectively. In the study by Zhou et al., the most common symptoms were fever and cough, respectively (13). In other studies, fever and respiratory symptoms were the most common symptoms in hospitalized patients (14, 16). Other studies also suggested that pneumonia and respiratory system involvement are the main complications of COVID-19 (4); however, the involvement of other organs is also reported in various studies (17-19).
Gastrointestinal symptoms in the studied patients with COVID-19 included nausea and vomiting (40%) and diarrhea (27.5%). In a study conducted in China, the prevalence of diarrhea and vomiting in patients with COVID-19 was 5% and 4%, respectively (13). Different studies reported a prevalence of 3% - 79% for gastrointestinal symptoms in patients with COVID-19 (20). Different prevalence of symptoms reported in various studies might be related to different populations, the diversity of drugs used in different regions, and the study time. Some studies showed that the prevalence of diarrhea increased since the onset of the COVID-19 epidemic (20). This can also be attributed to more focus on respiratory symptoms at the beginning of the epidemic, which expanded into other symptoms over time by a better understanding of the disease. Since the present study was performed on hospitalized patients, various criteria for the evaluation of patients in different regions could also explain the difference in the prevalence of gastrointestinal symptoms.
In the present study, about 30% of hospitalized patients reported a loss of sense of taste or smell. In a study in the United States, the prevalence of losing the sense of smell was 26% and 66.7% in hospitalized and non-hospitalized patients (21). In the present study, the prevalence of extrapulmonary symptoms- e.g., headache, loss of senses of taste and smell, were higher in patients who recovered from the disease. A study in the United States on 169 patients with COVID-19 revealed that patients who reported loss of sense of smell were less likely to need hospitalization (21).
According to the results of the current study, the correlation of BMI with disease outcome was different in the two groups, so that patients who died from COVID-19 had higher BMI values in the age group under 50, while the same subjects had lower BMIs in the age group above 50. A study on 3,615 patients with COVID-19 in New York City also showed that patients under 60 years with high BMI values were at a 2-fold risk for hospitalization and a 1.8-fold risk for admission to ICU; the study could not show any correlation between BMI and risk of hospitalization in patients aged above 60 (22). A meta-analysis has shown that obesity was associated with a significantly increased risk of critical COVID-19. The authors show that this association remained significant even after adjusting for several variables (23). Although the pathophysiology underlying COVID-19-infection has not been completely elucidated, many studies have suggested the role of the ACE2 receptor. In addition, studies show that obesity was accompanied by increased expression of ACE2 (24).
Regarding diabetes and immunodeficiency in the present study, the relative risk for mortality was~3; however, it was not statistically significant. Some studies showed that diabetes, hypertension, and chronic respiratory diseases are associated with death from COVID-19 (13). In agreement with our study, some other studies failed to find a significant relationship between COVID-19 and other risk factors e.g., diabetes and immunodeficiency. For example, in the study by Guo et al., although the mortality rate was higher in patients with COVID-19 and diabetes comorbidity compared with non-diabetic ones with COVID-19 (10.8% Vs. 3.6%), the difference between the groups was insignificant (14). Although many studies confirmed that diabetes and uncontrolled diabetes could be predisposing factors for some infectious diseases and death caused by them (25, 26), the results are contradicted in the case of COVID-19, and further studies are required.
5.1. Conclusion
Given the high prevalence of obesity in many countries, more attention should be paid to this dilemma as a risk factor that has received less attention thus far. In addition, the results of the present study confirmed that patients with more extrapulmonary symptoms are at a lower risk for death from COVID-19. Further studies in this regard are recommended.