1. Background
Coronaviruses belong to a large family of viruses and cause a range of diseases, including cold to more serious illnesses such as acute respiratory distress syndrome. Coronavirus disease 2019 (COVID-19) is a new type of coronavirus not previously observed in humans. The disease was identified for the first time in Wuhan, China (1). It is a dangerous disease that has led to the death of thousands of individuals in recent months, with an estimated rate of 2.8% - 20% in various studies (2, 3). The mortality rate of the virus was calculated at 3.4% by the World Health Organization (4).
Early symptoms include pneumonia, fever, muscle pain, and fatigue, which are similar to other viral illnesses, such as the flu (5). New diagnostic methods, including real-time polymerase chain reaction (RT-PCR), as the main approach to the diagnosis of COVID-19, and nucleic acid (NA) microarray-based measurements may be effective in monitoring and epidemiological measures, along with preventive measures. The COVID-19 NA is detectable in samples obtained from nasopharyngeal swabs, sputum, lower respiratory tract secretions, blood, and stool (5).
The development of safe and stable vaccines is a major challenge regarding COVID-19. At the moment, there is no antiviral or vaccine treatment for the infection. In such a sudden epidemic, scientists were unable to develop new drugs following traditional principles because it is a time-consuming process. Due to the necessity of developing an effective drug in the shortest possible time, it is necessary to consider the regular and extensive screening of existing drugs that are the main treatment options for other similar diseases, such as the flu (6).
The COVID-19 infection leads to impaired systemic and local respiratory problems that can result in a secondary infection for the treatment of which antibiotic therapy should be prescribed (7). Commonly, a standard three-drug protocol, including ribavirin, hydroxychloroquine, and lopinavir/ritonavir, is prescribed for patients with COVID-19. Ribavirin is administered if the patient shows symptoms such as decreased consciousness, respiratory rate (RR) of ≥ 24 breaths/min, blood pressure (BP) of < 90/60 mmHg, hypoxemia, and severe abnormalities in the Computed Tomography (CT) scan of the chest (8). However, some patients with COVID-19 do not respond to the standard three-drug protocol.
It seems that the risk of developing complications associated with COVID-19 is higher among individuals with weakened immune systems. In this regard, immunotherapy using immunoglobulin G (IgG) in combination with antiviral agents is recommended for the treatment of COVID-19 (9, 10). Intravenous Immunoglobulin (IVIG), as a well-known drug, has been applied to treat patients with autoimmune and chronic inflammatory diseases (11, 12). Intravenous immunoglobulin is also utilized against viruses, bacteria, and fungi in human patients (13, 14). The use of IVIG leads to a decrease in the production of cytokines, especially proinflammatory factors, and increases the inflammatory response (15).
2. Objectives
With this background in mind, the present study aimed to determine the effectiveness of IVIG for the treatment of patients with COVID-19. In addition, the current study assessed the effects of IVIG on COVID-19 patients not responding to the standard three-drug protocol in patients entering the intubation phase in comparison with those not entering the intubation phase.
3. Methods
This descriptive case-control study was performed on 26 patients with COVID-19 referring to Imam Reza hospital in Mashhad, Iran, in March 2020.
3.1. Inclusion and Exclusion Criteria
The inclusion criteria were the age range of 18 - 65 years, getting informed consent, diagnosis of COVID-19 based on RT-PCR, presence of at least one factor indicating the reduction of consciousness level, RR of ≥ 24 breaths/min, BP of < 90/60 mmHg, pulmonary infiltrations, hypoxemia, and no response to the standard three-drug protocol. The exclusion criteria were an allergy to IVIG, specific underlying diseases such as heart disease, the prohibition of IVIG, and special medical conditions not permitting the continuation of IVIG administration.
3.2. Study Design
In the present study, 26 patients diagnosed with COVID-19 were selected by purposive sampling. The presence or absence of the virus was confirmed using RT-PCR. The current study was conducted on 26 subjects with no response to the standard three-drug protocol, including ribavirin, hydroxychloroquine, and lopinavir/ritonavir, using purposive sampling. The data were collected from the medical records of COVID-19 patients at Imam Reza Hospital. The patients were included with severe symptoms of COVID not entering the intubation phase and subjects entering the intubation phase. In addition to the standard three-drug protocol, including ribavirin, hydroxychloroquine, and lopinavir/ritonavir, three doses (0.4 g/kg/day 25 g daily per 60 kg of body weight) of IVIG were administered for the patients. Intravenous immunoglobulin was routinely prescribed for critically ill patients not responding to the standard three-drug protocol.
A questionnaire, including the items regarding demographic characteristics, medical history, clinical characteristics, clinical examinations, laboratory findings, and mortality/recovery, was completed for all the patients. The presence or absence of the virus was confirmed using RT-PCR before and after the treatment. The subjects undergoing the prophylaxis of thrombosis received heparin in the absence of contraindication. The patients’ conditions were compared before and after the treatment.
3.3. Statistical Analysis
All the data were statistically analyzed using SPSS software (version 23). The normality of the variables was assessed by the one-sample Kolmogorov-Smirnov test. The quantitative and qualitative variables were compared by the independent t-test, Mann-Whitney U-test, and chi-square test. A P-value of less than 0.05 was considered statistically significant.
3.4. Ethical Considerations
The current study was approved by the Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran. The present study was carried out based on the guidelines of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use and the Helsinki Declaration. A code (code: IRCT20200325046859N1) was assigned to each included participant for observing data confidentiality. Written informed consent was obtained from all the patients, parents, or legal guardians. The subjects were assured of the confidentiality of their information. The patients received information about their clinical status. Moreover, the complications of each medication were expressed for the study participants. The cases were also ensured that they could withdraw from the study at any time.
4. Results
In general, 84.6% of the patients were males, and 15.4% of them were females. In this study, the mean age of the patients was 51.52 ± 16.4 years (age range: 26-90 years). Furthermore, the mean age was reported as 48.83 ± 18.8 and 54 ± 14.21 for the patients in the intubation phase and those not in the intubation phase, respectively. The comparison of the two groups in terms of age showed no significant difference between the groups (t = 0.79; P = 0.38). The mean Body Mass Index (BMI) was 31.44 ± 3.22 (BMI range: 25 - 39). In addition, the mean BMI was reported as 31.23 ± 3.19 and 31.67 ± 3.39 in patients not entering the intubation phase and those entering the intubation phase, respectively. The comparison of the two groups in terms of BMI showed no significant difference between the groups (t = 0.02; P = 0.88).
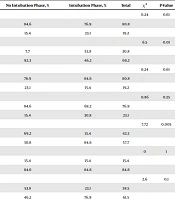
Table 1 compares patients in the intubation phase and those not in the intubation phase regarding primary symptoms, underlying diseases, social history, and drug history. Based on the obtained results of the current study, there was no significant difference between the two groups in terms of gender, primary outcomes except for chill and tachypnea, underlying diseases, social history, and drug history (P > 0.05). Table 2 compares patients in the intubation phase and those not in the intubation phase regarding laboratory findings. There was a significant difference between the two groups in terms of Red Blood Cell (RBC) count (P = 0.01), creatinine (P = 0.01), alkaline phosphatase (ALP) (P = 0.001), and platelet count (P = 0.02).
| Variable | No Intubation Phase, % | Intubation Phase, % | Total | χ2 | P-Value |
|---|---|---|---|---|---|
| Fever | 0.24 | 0.61 | |||
| Yes | 84.6 | 76.9 | 80.8 | ||
| No | 15.4 | 23.1 | 19.2 | ||
| Chill | 6.5 | 0.01 | |||
| Yes | 7.7 | 53.8 | 30.8 | ||
| No | 92.3 | 46.2 | 69.2 | ||
| Cough | 0.24 | 0.61 | |||
| Yes | 76.9 | 84.6 | 80.8 | ||
| No | 23.1 | 15.4 | 19.2 | ||
| Dyspnea | 0.86 | 0.35 | |||
| Yes | 84.6 | 69.2 | 76.9 | ||
| No | 15.4 | 30.8 | 23.1 | ||
| Tachypnea | 7.72 | 0.005 | |||
| Yes | 69.2 | 15.4 | 42.3 | ||
| No | 30.8 | 84.6 | 57.7 | ||
| Vomiting | 0 | 1 | |||
| Yes | 15.4 | 15.4 | 15.4 | ||
| No | 84.6 | 84.6 | 84.6 | ||
| Myalgia | 2.6 | 0.1 | |||
| Yes | 53.8 | 23.1 | 38.5 | ||
| No | 46.2 | 76.9 | 61.5 | ||
| Weakness and fatigue | 0 | 1 | |||
| Yes | 15.4 | 15.4 | 15.4 | ||
| No | 84.6 | 84.6 | 84.6 | ||
| Malnutrition | 0.24 | 0.61 | |||
| Yes | 15.4 | 23.1 | 19.2 | ||
| No | 84.6 | 76.9 | 80.8 | ||
| Headache | 0.96 | 0.32 | |||
| Yes | 0 | 7.7 | 4 | ||
| No | 100 | 92.3 | 96 | ||
| Muscle pain | 1.18 | 0.27 | |||
| Yes | 7.7 | 23.1 | 15.4 | ||
| No | 92.3 | 76.9 | 84.6 | ||
| Underlying diseases | 7.02 | 0.31 | |||
| None | 61.5 | 38.5 | 50 | ||
| Diabetes | 15.4 | 7.7 | 11.5 | ||
| Hypertension | 15.4 | 15.4 | 15.4 | ||
| Diabetes and hypertension | 7.7 | 15.4 | 3.8 | ||
| Liver transplant and hypertension | 0 | 7.7 | 7.7 | ||
| Heart disease | 0 | 15.4 | 3.8 | ||
| Digestive problems | 0 | 7.7 | 3.8 | ||
| Social history | 2 | 0.36 | |||
| None | 92.3 | 92.3 | 92.3 | ||
| Smoking | 0 | 7.7 | 3.8 | ||
| Addiction | 7.7 | 0 | 3.8 | ||
| Drug history | 5.61 | 0.46 | |||
| None | 61.5 | 46.2 | 53.8 | ||
| Anti-hypertensive | 15.4 | 15.4 | 15.4 | ||
| Anti-diabetic | 7.7 | 15.4 | 11.5 | ||
| Anti-hypertensive and anti-diabetic | 15.4 | 0 | 3.8 | ||
| Immunosuppressive | 0 | 15.4 | 7.7 | ||
| Heart medications and anti-hypertensive | 0 | 7.7 | 3.8 |
| Variable | No Intubation Phase, Mean ± SD | Intubation Phase, Mean ± SD | Total, Mean ± SD | t-test | P-Value |
|---|---|---|---|---|---|
| RBC, mL/mm3 | 5.17 ± 0.78 | - | 5.17 ± 0.78 | 7.14 | 0.01 |
| BUN, mg/dL | 34.09 ± 14.66 | 57.09 ± 31.64 | 45.59 ± 26.79 | 0.23 | 0.63 |
| Creatinine, µmol/L | 0.94 ± 0.12 | 1.47 ± 0.92 | 1.22 ± 0.71 | -2.47a | 0.01 |
| ESR, mm/h | 60.75 ± 26.41 | 81.14 ± 20.31 | 73.72 ± 23.72 | 0.63 | 0.23 |
| ALT, IU/L | 48.85 ± 50.84 | 44 ± 22.49 | 46.61 ± 38.85 | 1.11 | 0.31 |
| AST, IU/L | 51.57 ± 28.83 | 78.16 ± 71.77 | 63.84 ± 52.46 | 1.85 | 0.2 |
| ALP, IU/L | 189 ± 52.41 | 272.1 ± 137.51 | 244.4 ± 120.24 | 25.3 | 0.001 |
| Bilirubin, µmol/L | 0.52 ± 0.25 | - | 0.52 ± 0.25 | -b | -b |
| Direct bilirubin, µmol/L | 0.2 ± 0.15 | - | 0.2 ± 0.15 | -b | -b |
| LDH, U/L | 665.5 ± 195.64 | 504 ± 213.62 | 61.67 ± 207.66 | 0.05 | 0.81 |
| INR | 1.22 ± 0.306 | - | 1.22 ± 0.306 | -b | -b |
| Na, mmol/L | 137.3 ± 3.67 | 127 ± 4.2 | 135.8 ± 5.18 | 0.02 | 0.88 |
| K, mmol/L | 4.06 ± 0.804 | 3.96 ± 0.057 | 4.04 ± 0.71 | 1.33 | 0.26 |
| PT | 14.08 ± 2.806 | - | 14.08 ± 2.8 | -b | -b |
| PTT | 33.07 ± 5.509 | - | 33.07 ± 5.5 | -b | -b |
| O2sat | 82.75 ± 6.45 | 88.10 ± 3.957 | 85.18 ± 5.997 | 4.204 | 0.054 |
| WBC, × 1000 mL | 8.38 ± 3.22 | 11.91 ± 20.01 | 10.15 ± 14.16 | -1.15 | 0.24 |
| Lymphocyte count, % | 22.04 ± 24.28 | 10.76 ± 5.03 | 16.87 ± 18.75 | -2.34 | .019 |
| Neutrophil count | 80.35 ± 6.58 | 84.83 ± 6.98 | 82.408 ± 7.001 | 0.04 | 0.844 |
| Hemoglobin | 13.75 ± 1.86 | 13.707 ± 1.79 | 13.73 ± 1.79 | 0.04 | 0.83 |
| Platelet count | 248.15 ± 73.96 | 153.17 ± 38.34 | 202.56 ± 75.86 | 5.98 | 0.02 |
| CRP | 132.225 ± 73.94 | 116.26 ± 89.82 | 124.59 ± 80.42 | 1.08 | 0.31 |
Abbreviations: ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate transaminase; BUN, blood urea nitrogen; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; INR, international normalized ratio; K, potassium; LDH, lactic acid dehydrogenase; Na, sodium; PT, prothrombin time; PTT, partial thromboplastin time; O2sat, oxygen saturation; RBC, red blood cell; WBC, white blood cell.
aMann-Whitney U-test.
bIncomputable.
The lymphocyte count range significantly increased after IVIG therapy in patients not entering the intubation phase (Z = -2.06; P = 0.03) while no difference was observed in patients in the intubation phase before and after IVIG therapy (Z = -1.22; P = 0.22). Moreover, C-reactive Protein (CRP) significantly decreased after IVIG therapy in patients not entering the intubation phase (t = 4.4; P = 0.001) while no difference was reported in patients in the intubation phase before and after IVIG therapy (t = -0-802; P = 0.45).
The mean hospital stay was 8.22 ± 2.7 (range: 5 - 14) and 15.14 ± 6.7 (range: 7 - 27) days in patients in the intubation phase and those not in the intubation phase, respectively. The comparison of patients in the intubation phase and those not in the intubation phase regarding the duration of hospital stay showed a significant difference between the two groups (t = 6.34; P = 0.02). The mean ICU stay was 1.46 ± 1.8 (range: 0 - 5) and 15 ± 4.2 (range: 10 - 22) days among patients in the intubation phase and those not in the intubation phase, respectively. The comparison of patients in the intubation phase and those not in the intubation phase regarding the duration of ICU stay showed a significant difference between the two groups (t = 14.05; P = 0.001). The patients received IVIG within 3-5 days after admiration to the hospital.
Table 3 compares the laboratory findings after receiving IVIG in patients in the intubation phase and those not in the intubation phase. The obtained results of the present study showed that there was a significant difference between the two groups in terms of lymphocyte count (P > 0.005). However, a difference was detected between the two groups in terms of oxygen saturation (O2sat), White Blood Cell (WBC), hemoglobin level, and neutrophil count.
| Variable | No Intubation Phase, Mean ± SD | Intubation Phase, Mean ± SD | Total, Mean ± SD | t-test | P-Value |
|---|---|---|---|---|---|
| O2sat | 94 ± 2.23 | 94.5 ± 2.082 | 94.12 ± 2.147 | 0.279 | 0.605 |
| WBC, × 1000 mL | 17.72 ± 24.41 | 11.75 ± 7.62 | 14.73 ± 17.97 | -0.07 | 0.93 |
| Lymphocyte count, % | 32.91 ± 15.92 | 8.22 ± 5.6 | 21.06 ± 17.31 | -3.75 | < 0.005 |
| Neutrophil count | 73.08 ± 19.13 | 84.96 ± 9.24 | 78.52 ± 16.27 | 3.193 | 0.088 |
| Hemoglobin | 13.1 ± 1.56 | 10.73 ± 1.91 | 11.91 ± 2.09 | 0.51 | 0.47 |
| Platelet count | 326.33 ± 118.906 | 138.67 ± 105.95 | 232.5 ± 146.01 | 1.18 | 0.28 |
| CRP | 53.167 ± 35.64 | 105.61 ± 55.28 | 70.65 ± 48.66 | 1.448 | 0.25 |
Abbreviations: CRP, C-reactive protein; O2sat, Oxygen saturation; WBC, white blood cell.
The comparison of the laboratory findings before and after receiving IVIG in patients in the intubation phase and those not in the intubation phase showed a significant difference between patients in the intubation phase and those not in the intubation phase in terms of oxygen saturation (O2sat) (Z = -6.24; P < 0.005), WBC (Z = -3.17; P = 0.001), and hemoglobin level (t = 3.5; P = 0.002). Moreover, a significant difference was observed in the CRP level (t = 2.23; P = 0.04). However, no difference was detected between patients in the intubation phase and those not in the intubation phase in terms of lymphocyte count (Z = -1.5; P = 0.13), neutrophil count (t = 1.5; P = 0.14), and platelet count (Z = -1.49; P = 0.14).
In general, 14 (53.8%) patients were discharged, and 12 (46.2%) subjects expired. All the study participants in the pre-intubation phase were recovered; nevertheless, only one patient (7.7%) who received IVIG after entering the intubation phase was recovered, and the other 12 subjects (92.3%) died. The comparison of patients in the intubation phase and those not in the intubation phase in terms of mortality showed a significant difference between the groups (χ2 = 22.28; P < 0.005).
5. Discussion
In summary, the present study confirmed the therapeutic effects of IVIG in patients with COVID-19. The comparison of the laboratory findings before and after receiving IVIG showed the patients’ recovery regarding O2sat, WBC, hemoglobin levels, and CRP level; however, no difference was observed in terms of lymphocyte counts and neutrophils counts. Furthermore, there was a difference between patients in the intubation phase and those not in the intubation phase respecting RBC, creatinine, AST, lymphocyte counts, and platelet counts. The lymphocyte count range increased, and CRP decreased after IVIG therapy in patients not entering the intubation phase, while no difference was observed in patients entering the intubation phase before and after IVIG therapy. All the patients not entering the intubation phase were recovered; however, only one patient entering the intubation phase was recovered, and other subjects expired.
There are several options for the treatment of patients with COVID-19. Chloroquine and remdesivir are the most commonly used drugs for the treatment of patients with COVID-19 (16). The addition of anti-cytokine biological agents to the standard treatment protocol of COVID-19 is suggested considering the pathogenesis of the virus causing the cytokine storm (17). Anakinra (an interleukin-1 receptor antagonist) and tocilizumab (an interleukin 6 [IL-6] receptor antagonist) are two anti-cytokine biological agents commonly used in rheumatological autoimmune inflammatory conditions that can be suggested for COVID-19 patients experiencing cytokine storms. The IVIG is another proposed agent containing a panoply of antiviral antibodies. Clinically, IVIG, known as an adjunctive drug for severe pneumonia caused by influenza, is commonly used in the treatment of critical patients. Based on the literature, the effectiveness of IVIG (composed of extracted immunoglobulin from healthy individuals) is confirmed for the treatment of cases with macrophage activation syndrome and septic shock (17, 18). Based on an animal study, IVIG decreases the inflammation of intestinal epithelial cells and stops the development of Candida albicans as the opportunistic human fungal pathogen (19). The IVIG can regulate proinflammatory mediators and anti-inflammatory cytokines (20).
The protein is rich in bacterial antibodies and viral IgG; accordingly, its continuous infusion may lead to the improvement of the IgG level in the serum. It can lead to neutralizing the pathogens in the respiratory tract of patients and consequently shortening the course of disease via promoting the body’s defense system and preventing further damage to the target cells. Moreover, the process of lymphocyte differentiation and maturation can be affected by the use of IVIG. This leads to improving the normal immune response of white blood cells and controlling the inflammatory factors (21, 22).
The effects of moderate-dose corticosteroids (160 mg/day) with immunoglobulin (20 g/day) in patients with COVID-19 was assessed in a study carried out by Zhou et al. (23) Based on the obtained results, the improvements of the Acute Physiology and Chronic Health Evaluation score, temperature, lymphocyte count, and CRP were observed after the treatment. In addition, there was an improvement in the oxygen supply index (SpO2 and PaO2/FiO2). The CT scan of the chest showed that lung lesions clearly improved in the majority of patients. In the aforementioned study, it was also concluded that moderate-dose corticosteroids, along with immunoglobulin, are effective in the treatment of COVID-19 patients (23). Similarly, in another study carried out by Khodashahi et al. (24), three patients with COVID-19 with no response to the standard three-drug protocol were treated with a high dose of IVIG (0.4 g per kg body weight per day for 3 - 5 days; total: 25 g). The chest CT scans of the three subjects were completely normal after adding IVIG to the drug regimen. The results of the aforementioned study are in line with the findings of other similar studies carried out by Cao et al. (25) and Ni et al. (26).
Similar to the present study, one multicenter retrospective cohort study conducted by Shao et al. (27) investigated the clinical efficacy of IVIG therapy in patients with COVID-19. The IVIG was administered to 174 cases out of 325 patients. The obtained results indicated a lower lymphocyte count and oxygenation index, as well as higher levels of IL-6 plasma and lactate, in the IVIG group than in the other group (27). Likewise, in the present study, the lymphocyte count decreased following IVIG therapy.
The effectiveness of regular IVIG therapy in the prognosis of COVID-19 patients with severe pneumonia was assessed in a study by Xie et al. (28). Among 58 included patients with severe COVID-19 undergoing IVIG therapy, 39.6% of the subjects died within 28 days. Shao et al. showed no difference between the IVIG-receiving and control groups in terms of 28-day and 60-day mortalities. However, the use of IVIG could reduce the 28-day mortality in critical patients, and the application of a high dose of IVIG in the early stage (i.e., less than seven days after admission) led to the reduction of 60-day mortality in critical patients.
Similarly, the improved inflammatory response and some organ functions due to using IVIG were reported in patients with the critical type. Therefore, in the aforementioned study, it was concluded that the early administration of a high dose of IVIG resulted in better outcomes in patients with critical COVID-19 (27). This was confirmed by the findings of the current study on the mortality rate of patients not entering the intubation phase. In this study, there was also a lower rate of mortality and better outcomes in subjects treated in the critical phase (i.e., not entering the intubation phase) in comparison with those reported for patients not entering the intubation phase.
In this regard, the right time for the selection of antiviral therapy is very important. There is an association between the phase of the disease and the recovery of patients undergoing IVIG therapy. The critical time of treatment with IVIG, namely when the potent suppression of inflammatory cascade occurs, is very important. At this time, the patients can be protected from fatal immune-mediated injuries in the first few days of deterioration by blocking the progression of the COVID-19 cascade (29).
The obtained results of the present study showed that the best outcomes were observed when IVIG was administered in the first stage of the disease; nevertheless, it may have no benefit in patients with developed systemic damage who enter the intubation phase. The IVIG can be considered a safe treatment for patients with COVID-19 at the early stage of the disease. However, the potential cardiovascular or renal diseases should be taken into account during the administration of IVIG. The IVIG has been previously used in an epidemic named the West Nile Fever. The condition was controlled by an IVIG generation extracted from a healthy Israeli convalescent blood population (30-32). The agent can help control infectious conditions by the transfer of a normal innate immune system of the healthy population to the infected patients (33, 34).
The action mechanism of IVIG has not been completely perceived. The immune response is modulated by IVIG, which may act by blocking an extensive range of proinflammatory cytokines, Fc-gamma receptors, and leukocyte adhesion molecules. Moreover, the function of IVIG may be due to the suppression of pathogenic subsets of T helper cells type 1 and type 17, as well as the neutralization of pathogenic autoantibodies (35, 36). The F2 fragment and crystallizable fragment are two functional portions of IgG antibodies, which play the main role in the activation of the immune response (37). Immunotherapy with immune IgG antibodies combined with antiviral drugs is suggested as an alternative treatment for newly infected patients with COVID-19. This method functions the best when the immune IgG antibodies are collected from COVID-19 subjects who have improved in the same region (19). Probably, the infusion of plasma from recovered COVID-19 donors can increase the anti-inflammatory characteristics of dendritic cells. This issue is very important in subjects with COVID-19 in excessive inflammatory phases (38).
Immunomodulation properties of IVIG may be important in the prevention and management of COVID-19. The IVIG helps enhancement of immunogenicity and has positive effects on the symptoms of COVID-19. Besides, IVIG enhances innate immunity against COVID-19 and influences the cytokine production and activation of immune effector cells. The adverse events of IVIG are associated with specific immunoglobulin preparations and individual differences that can be minimized with changing from IVIG to subcutaneous immunoglobulin (39-41). The IVIG at a dose of 2 g per kg body weight for four days is suggested to lower side effects (42). It is not necessary to mention that the use of IVIG produced from a large number of sera of convalescent subjects with viral infections has been reported with better outcomes that could be administered at smaller doses (43-46).
5.1. Conclusions
In summary, the results of the present study confirmed the therapeutic effects of IVIG in patients with COVID-19. Moreover, better treatment results, shorter hospital stay, and lower mortality rates were observed among COVID-19 patients who did not enter the intubation phase in comparison with those entering the intubation phase.