1. Background
Bacterial meningitis infections are prevalent worldwide and are considered a serious public health problem because of their magnitude, transmission potential, pathogenicity, and social relevance (1). Despite the reduced incidence of the disease after introducing a vaccine against its main causative agents, bacterial meningitis is still significantly responsible for high morbidity and mortality in children globally (2, 3). Word wild mortality rates remain extremely high, ranging from 5 to 30% of cases, and approximately 50% of survivors evolve with neurological sequelae (4). According to the World Health Organization, every year, bacterial meningitis epidemics affect more than 400 million people living in the 26 countries of the extended "African meningitis belt," ranging from Senegal to Ethiopia. As a result, they cause death in 10% of bacterial meningitis cases and a 10 - 20% increase in neurological sequelae (5).
In Brazil, in 2018, 6,441 cases of bacterial meningitis and 1,104 deaths from the disease were registered, and the lethality varied between 9 - 31% according to the etiological agent (6). According to the latest data released by the State Secretariat, 424 cases of meningitis were confirmed in the first half of 2015 in the state of Minas Gerais, corresponding to an incidence rate of 2.03/100,000 inhabitants. In the same period, 70 deaths were registered with a lethality rate of 16.5% (7).
Among children surviving an episode of bacterial meningitis, up to 50% reported sequelae ranging from sensorineural hearing loss to motor deficits, epilepsy, developmental delay, and poor school performance (4, 8, 9). In pediatric care, these deficits are potentially more impactful, as even a focal loss can impair the child's interaction with the environment, further impairing the acquisition of sensorimotor skills and neurocognitive development that can persist until adulthood.
Identifying risk factors causing severe outcomes in children diagnosed with meningitis helps define patients who could benefit from aggressive therapeutic interventions to reduce the unfavorable prognosis. Several risk factors associated with prognosis in childhood bacterial meningitis have been previously described. However, the findings of different studies are conflicting or inconclusive due to either the limited number of patients or the inclusion of patients with laboratory and clinical findings suggestive of meningitis but lacking isolation of the etiologic agent using microbiological or molecular tests (4, 10-14).
2. Objectives
This study aimed to define risk factors associated with suppurative complications, short-term sequelae, and death in patients with confirmed bacterial meningitis in the state of Minas Gerais, Brazil.
3. Methods
3.1. Delineation and Location
This was a retrospective cohort study carried out from January 2005 to December 2018 at the Hospital Infantil João Paulo II (HIJPII) of Fundação Hospitalar de Minas Gerais (FHEMIG). The hospital offers free public health care services for pediatric patients and is a reference for treating infectious diseases in Minas Gerais state, Brazil.
3.2. Inclusion and Exclusion Criteria
This study included patients aged 0 - 18 years, diagnosed with bacterial meningitis, and admitted to the service during the study period. Patients whose information could not be retrieved were excluded from the study.
3.3. Sample
A sample size of at least 150 children would obtain 90% statistical power in estimating the percentage of death, considering that this value in the population would be 13.9% (15) with 10% precision.
3.4. Definitions
The diagnosis of bacterial meningitis was defined by (1) chemocytological changes in the cerebrospinal fluid (CSF), such as a decrease in glucose level below 2/3 of capillary blood glucose and an increase in protein and leukocytes, with a predominance of polymorphonuclear cells; and (2) isolation of etiologic agents through culture, latex binding test, or polymerase chain reaction in CSF or peripheral blood samples. Reference values considered normal for assessing newborns were < 150 mg/dL for protein and < 25 cell/mL for leukocytes. For patients over 1 month of age, protein values < 40 mg/dL and leukocytes values < 10 cell/mL were considered normal.
3.5. Data Collection
Data collection was performed using a standardized questionnaire completed through information obtained from medical records and notification forms from the Acute Notification Information System. Relevant data included age, gender, vaccination status, exposure to people diagnosed with meningitis, traveling two weeks preceding onset of symptoms, previous use of antibiotics, signs and symptoms on admission, and laboratory and imaging test results such as chemocytological profile of CSF, treatment received, complications, and patient outcome. The patients were also stratified into three groups according to etiological agents: Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae. The patients’ clinical and laboratory characteristics were studied according to the identified etiologic agent.
We studied predictor variables associated with severe outcomes in pediatric patients with bacterial meningitis. Signs and symptoms considered as predictor variables were: (1) seizures; (2) motor deficits; (3) osteoarticular symptoms; (4) respiratory symptoms, including flu-like symptoms, cough, odynophagia, otalgia, and otorrhea; (5) gastrointestinal symptoms, such as diarrhea, bloating, and abdominal pain; (6) intracranial hypertension identified through arterial hypertension, bradycardia, respiratory arrhythmia (Cushing's triad), and papilla edema; (7) hemorrhagic manifestations defined as the presence of petechiae, suffusions, or bleeding; and finally (8) signs of clinical severity defined as findings suggestive of hemodynamic instability such as slowed capillary perfusion, hypotension, bradycardia, groaning, cold extremities, paleness, and hypoxia.
Severe outcomes were defined as: (1) suppurative complications during hospitalization (abscess or empyema diagnosed by neuroimaging); (2) survival associated with sequelae during hospital discharge; and (3) death. The following were defined as sequelae: hydrocephaly requiring ventriculoperitoneal shunt, epileptic seizure requiring outpatient use of anticonvulsant, motor deficit or cognitive deficit, and hearing deficit diagnosed by otorhinolaryngological evaluation. Any evidence of general learning difficulty regarding academic capacity and adaptive behavior that influenced the child's capacity for autonomy, compared to incidents reported by the family prior to the infectious event, was considered a cognitive deficit. The diagnosis of hydrocephalus was performed through imaging studies of the central nervous system such as computed tomography and magnetic resonance imaging.
3.6. Statistical Analysis
The analyses were performed using the Statistical Package for Scientific Science (SPSS) software, version 21. For descriptive analysis, we determined frequency, percentage, and measures of central tendency. Demographic, clinical, and laboratory characteristics were compared between the three groups of leading etiologies using X2 or Fisher's exact test for categorical variables and Student's t-test, analysis of variance, or Kruskal-Wallis tests for continuous variables.
Logistic regression analysis was performed to identify independent risk factors associated with severe outcomes. Univariate analysis was performed by estimating odds ratios (ORs) and 95% confidence intervals. A preliminary selection of variables, including those with P < 0.20, was made for multivariate regression analysis. The significance level was P < 0.05 for the final model.
3.7. Ethical Considerations
This study was evaluated and approved by the Teaching and Research Committees of FHEMIG and the Federal University of Minas Gerais.
4. Results
During the cohort period, 1,468 patients with meningitis were admitted to HIJPII. Of them, 840 had bacterial etiology defined according to the laboratory characteristics of CSF. It was possible to identify the etiological agent in 178 cases. For the following analysis, only bacterial meningitis with a confirmed etiologic agent was considered.
Among the patients, 93 (52%) were male and 85 (48%) were female. The age of the patients ranged from 0 months to 17 years with a median of 43.4 months, and the predominant age group was under one year (36%). Meningococcal meningitis was the most prevalent disease form accounting for 91 (51%) cases, followed by pneumococcal meningitis in 53 (31%) patients and H. influenzae type B meningitis in 18 (10%) patients. Other bacteria identified were Streptococcus agalactiae (2), Klebsiella pneumoniae (1), Pseudomonas aeruginosa (1), Streptococcus pyogenes (1), and Salmonella sp. (1).
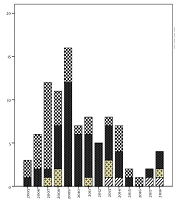
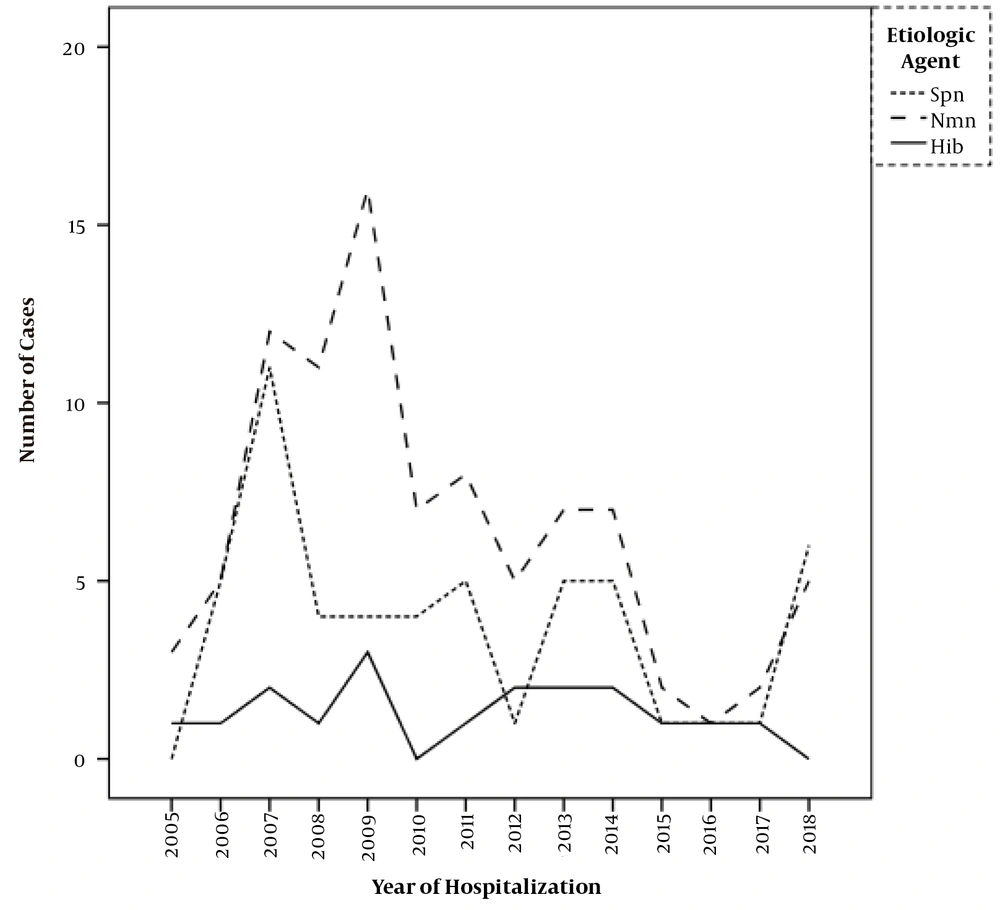
As shown in Figure 1, there was a trend toward stability in the number of meningitis cases caused by H. influenzae type B over the cohort years. A reduced frequency of occurrence was noted with N. meningitidis and S. pneumoniae, especially after 2010. N. meningitidis showed a 48% reduction from an average of 9.4 cases/year between 2005 and 2009 to 4.9 cases/year between 2010 and 2018. Streptococcus pneumoniae showed a 30% reduction from an average of 4.8 cases/year in the first period to 3.4 cases/year in the second period.
Among the 178 cases, 99 (55,6%) had the etiologic agent identified through culture analysis in a sample of cerebrospinal fluid and/or blood, 37 had pneumococcal meningitis, 47 had meningococcal, and 15 had H. influenzae. An antibiogram was performed for all these patients. One sample was identified with penicillin-resistant pneumococcus, with an MIC of 32 µg/mL (16). No ceftriaxone-resistant pneumococci were identified among the analyzed samples. The 79 remaining cases had the identification of the etiological agent through latex or molecular test, and it was not possible to perform an antibiogram.
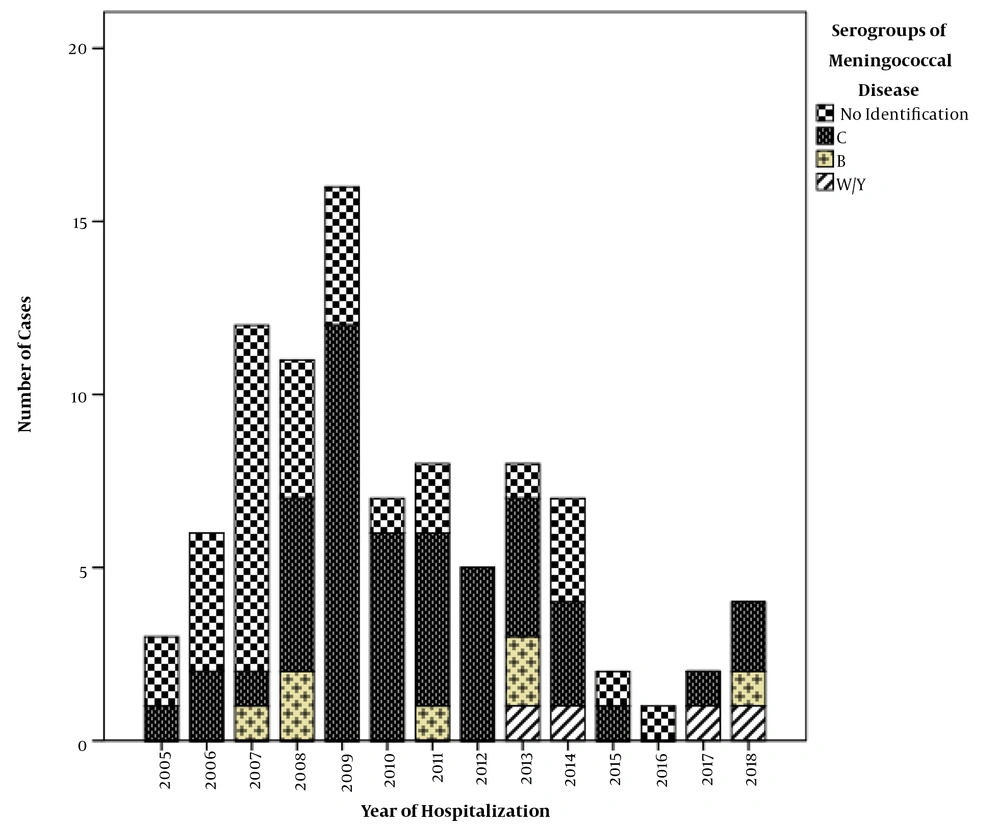
Serogroups associated with the meningococcal disease could be identified in 60 (66%) of 91 cases. Serogroup C was the most prevalent (82%; n = 49), followed by serogroup B (12%; n = 7) and serogroup WY (6%; n = 4). The emergence of serogroups W and Y occurred most clearly from 2013 onwards, as shown in Figure 2.
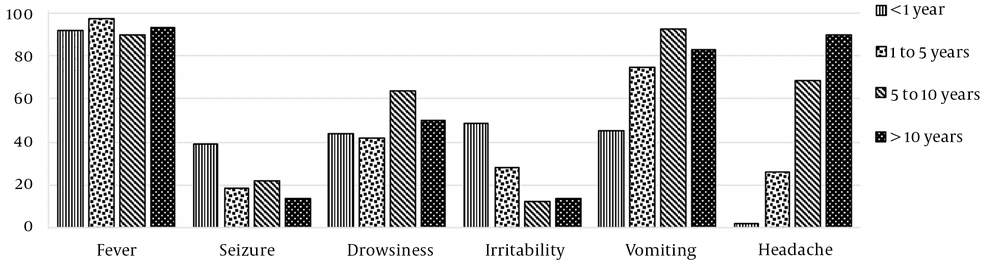
The main clinical findings at admission were fever (93.1%), vomiting (67.9%), drowsiness (47.8%), headache (36.5%), irritability (28.3%), and seizures (26.4%). Convulsion and irritability were more prevalent in children under one year of age, while vomiting and headache became more evident in older children, as shown in Figure 3.
Among the infants under one year of age, 39% had fontanel bulging. In children over one year of age, 63% had neck stiffness, 42% showed positive Brudzinski's sign, and 35.2% showed positive Kerning's sign. Moreover, 27% of the patients did not present any of the meningeal signs, and there was a statistically significant correlation between younger age and the absence of these findings (P < 0.001). The mean age of patients who did not present any of the meningeal signs was 34 months (St.d. deviation 44.6) versus 63 months (St.d. deviation 53.5) in the group with at least one of the physical examination findings.
Treatment with penicillin was instituted in 49 (27.5%) of the patients. Ceftriaxone monotherapy was used in 120 (67.4%) of the patients and associated with the second antibiotic in eight (4.5%) of the patients. As an association, vancomycin was used in suspected antimicrobial resistance, and chloramphenicol or doxycycline was used for the empirical treatment of hemorrhagic fevers.
Suppurative complications, including diagnoses of subdural empyema, brain abscess, septic sinus thrombosis, and ventriculitis, were recorded in 33 (19%) of the patients. The prevalence of neurological sequelae during hospital discharge was 12.4% (n = 22). Hearing (41%; n = 9) and cognitive (9%) impairments were prominent among the diagnosed sequelae. Incidences of epileptic crises, vestibular disorders, or hydrocephalus were noted in one case each (4.5%). Of the 178 patients, 22 (12.4%) died. The mechanism of death in these patients was predominantly hemodynamical (55%; n = 12) and neurological, with a diagnosis of brain death or neurogenic shock in 45% of the cases (n = 10).
A comparison of the patients' demographic, clinical, and laboratory characteristics according to the main etiologies is described in Tables 1 and 2.
| Chemocytological Characteristics of CSF | Etiologic Agent | P | ||
|---|---|---|---|---|
| Streptococcus pneumoniae | Neisseria meningitidis | Haemophilus influenzae Type B | ||
| Glucose level (mg/dL) | ||||
| Median - IIQ: 25 - 75% | 16 (1 - 36) | 20 (5 - 46) | 20 (16.8 - 24) | 0.27 |
| Protein (mg/dL) | ||||
| Median - IIQ: 25 - 75% | 250 (161 - 378.5) | 260 (124.3 - 388.1) | 177 (99.6 - 241.3) | 0.11 |
| Leukocytes (cel/mm3) | ||||
| Median - IIQ: 25 - 75% | 445.0 (96 - 931) | 1900 (790 - 5970) | 588.5 (211 - 2775) | 0.00 |
Comparison of the Chemocytological Characteristics of Cerebrospinal Fluid According to the Etiologic Agent of Bacterial Meningitis (2005 - 2018)
| Characteristics | Sequel | P | Suppurative Complications | P | Death | P | |||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | ||||
| Gender | |||||||||
| Male | 10 | 77 | 17 | 76 | 12 | 81 | |||
| Female | 12 | 71 | 16 | 69 | 10 | 75 | |||
| Total | 22 | 148 | 0.364 | 33 | 145 | 0.539 | 22 | 156 | 0.5 |
| Age | |||||||||
| < 1year | 14 | 48 | 26 | 38 | 13 | 51 | |||
| 1 - 5 years | 1 | 38 | 4 | 39 | 4 | 39 | |||
| 5 - 10 years | 4 | 36 | 2 | 39 | 4 | 37 | |||
| > 10 years | 3 | 26 | 1 | 29 | 1 | 29 | |||
| Total | 22 | 148 | 0.025 | 33 | 145 | < 0.001 | 22 | 156 | 0.02 |
| Etiologic agent | |||||||||
| Streptococcus pneumoniae | 12 | 38 | 13 | 40 | 10 | 43 | |||
| Neisseria meningitidis | 5 | 82 | 8 | 83 | 9 | 82 | |||
| Haemophilus influenzae type B | 5 | 12 | 5 | 13 | 1 | 17 | |||
| Total | 22 | 132 | 0.002 | 26 | 136 | 0.016 | 20 | 156 | 0.187 |
| Seizures | |||||||||
| No | 19 | 113 | 17 | 115 | 10 | 122 | |||
| Yes | 9 | 35 | 16 | 30 | 12 | 34 | |||
| Total | 22 | 148 | 0.075 | 33 | 145 | 0.002 | 22 | 156 | 0.002 |
| Motor deficits | |||||||||
| No | 14 | 123 | 23 | 121 | 18 | 126 | |||
| Yes | 8 | 25 | 10 | 24 | 4 | 30 | |||
| Total | 22 | 148 | 0.037 | 33 | 145 | 0.063 | 22 | 156 | 0.586 |
| Osteoarticular symptom | |||||||||
| No | 22 | 145 | 0 | 3 | 21 | 154 | |||
| Yes | 0 | 3 | 33 | 142 | 1 | 2 | |||
| Total | 22 | 148 | 0.658 | 33 | 145 | 0.538 | 22 | 156 | 0.328 |
| Respiratory symptoms | |||||||||
| No | 19 | 141 | 29 | 139 | 20 | 148 | |||
| Yes | 3 | 7 | 4 | 6 | 2 | 8 | |||
| Total | 22 | 148 | 0.124 | 33 | 145 | 0.091 | 22 | 156 | 0.357 |
| Gastrointestinal symptoms | |||||||||
| No | 20 | 140 | 3 | 8 | 18 | 149 | |||
| Yes | 2 | 8 | 30 | 137 | 4 | 7 | |||
| Total | 22 | 148 | 0.380 | 33 | 145 | 0.33 | 22 | 156 | 0.033 |
| Intracanial hypertension | |||||||||
| No | 21 | 147 | 33 | 141 | 21 | 153 | |||
| Yes | 1 | 1 | 0 | 4 | 1 | 3 | |||
| Total | 22 | 148 | 0.243 | 33 | 145 | 0.437 | 22 | 156 | 0.413 |
| Hemorrhagic manifestations | |||||||||
| No | 21 | 122 | 1 | 27 | 16 | 134 | |||
| Yes | 1 | 26 | 32 | 118 | 6 | 22 | |||
| Total | 22 | 148 | 0.098 | 33 | 145 | 0.016 | 22 | 156 | 0.105 |
| Sensory change | |||||||||
| No | 21 | 141 | 0 | 9 | 21 | 148 | |||
| Yes | 1 | 7 | 33 | 136 | 1 | 8 | |||
| Total | 22 | 148 | 0.723 | 33 | 145 | 0.151 | 22 | 156 | 0.692 |
| Clinical severity | |||||||||
| No | 15 | 124 | 8 | 23 | 14 | 133 | |||
| Yes | 7 | 24 | 25 | 122 | 8 | 23 | |||
| Total | 22 | 148 | 0.076 | 33 | 145 | 0.184 | 22 | 156 | 0.019 |
Comparative Analysis of the Characteristics of Hospitalized Patients with Bacterial Meningitis According to the Sequel to Hospital Discharge, Suppurative Complications, and Death
The convulsive crisis showed an association with S. pneumoniae (P < 0.001), while hemorrhagic manifestations, defined as the presence of petechiae, suffusions, or bleeding, were associated with N. meningitidis (P < 0.001). Increased cellularity was most significantly observed in patients with meningococcal meningitis, with a median of 1900 cells/mm3 (IIQ: 25 - 75%, 790 - 5970; P < 0.001) (Table 1).
Univariate analysis of predictive factors for neurological sequelae at hospital discharge revealed that age less than one year (P = 0.025), S. pneumoniae (P = 0.002), and presence of motor deficits at admission (P = 0.037) were all related to higher risk for neurological sequelae. In multivariate analysis, there was an association between the etiological agents S. pneumoniae (P = 0.006) and H. influenzae type B (P = 0.004) with a relative risk (RR) of 5.18 in patients with N. meningitidis. Eight patients from the cohort were not included in the sequelae analysis due to the unavailability of information on the outcome.
Univariate analysis of predictive factors for suppurative complications showed that age less than one year (P < 0.001), S. pneumoniae (P = 0.016), convulsive crisis (P = 0.002), and hemorrhagic manifestations (P = 0.016) were defined as risk factors. Age less than one year during diagnosis [P = 0.008; RR 16.26 (95% CI, 2.06 - 128.6)] and seizures [P = 0.038; RR 2.53 (95% CI, 1.05 - 6.08)] were independent risk factors for suppurative complications after multivariate analysis.
In univariate analysis, the deceased patients were children under one year of age (P = 0.02) who had low CSF cellularity (P = 0.013), with a median of 198 cells/mm3 (IIQ 25 - 75%, 38 - 800), seizures (P = 0.002), gastrointestinal symptoms (P = 0.033), and signs of clinical severity (P = 0.019) during admission. Also, there was an association between the use of ceftriaxone in monotherapy and death in patients who received penicillin (P = 0.046).
After multivariate analysis, independent risk factors for death were gastrointestinal symptoms [P = 0.02; RR 5.066 (95% CI, 1.29 - 19.87)], along with signs of clinical severity [P = 0.015; RR 3.453 (1.27 - 9.39)]. The comparative data of the univariate analyses and the patients' characteristics according to the outcomes are summarized in Table 2.
5. Discussion
The cohort in question is extremely important because this is the first study that included such a significant sample of pediatric patients, all with confirmed etiological agents. HIJPII is responsible for the care of 20% of meningitis cases in children from Minas Gerais, a second-most populous state in Brazil. It allows us to assume that both epidemiological description and evaluation of risk factors associated with unfavorable clinical outcomes satisfactorily characterize the bacterial meningitis scenario. In addition, studies with defined bacterial agents are not frequent, and other microorganisms, including viruses, and liquor alterations due to systemic response inflammation to infection could interfere in some published data (4, 10-14).
By analyzing the distribution of identified etiological agents, a trend toward stability was noticed in the number of cases over the years concerning H. influenzae type B, probably because the study started after developing a conjugate vaccine against the etiological agent in the National Immunization Program (NIP). Vaccines for the etiological agents N. meningitidis and S. pneumoniae were developed in 2010. After that, a trend toward reduction was recorded in the incidence of meningitis (17).
Regarding cases of meningococcal meningitis, there was a significant predominance of serogroup C, which was responsible for 84% of the cases identified in this cohort. It was also the main serogroup responsible for meningococcal disease in Brazil (18, 19). The distribution of the cases according to the remaining serogroups is concurrent with the data from the State of São Paulo, which has the highest reported rates of meningococcal disease in the country with a predominance of serogroup C (82.5%), followed by serogroups B (10.9%), W (6%), and Y (1.2%) (19). Although meningococcus C is arguably responsible for the vast majority of meningococcal meningitis cases in Brazil, making the vaccine exclusive to serogroup C responsible for reducing the total number of cases of the disease, we should be aware of the proportional increase in serogroups not included in the NIP.
The clinical presentation of bacterial meningitis in children varies depending on the patient's age and the time of disease evolution. Fever was the most prevalent clinical finding in the cohort of patients. Convulsive crises and irritability predominated in children under one year of age. This data is concordant with previous studies showing that about 20 - 50% of infants presented a feverish picture as the initial manifestation of infection, associated with non-specific symptoms and convulsive crises (3). In older children, the classic triad of fever, headache, and vomiting becomes more expressive, and about 20% of patients present convulsive crises prior to diagnosis (3, 20).
The reliability of meningeal signs in physical examination for the diagnosis of bacterial meningitis in childhood has been addressed numerously in previous studies. A systematic review published in 2015 evaluated prospective studies in children with suspected bacterial meningitis. It showed sensitivity of 51% for neck stiffness, 66% for Brudzinski's sign [positive predictive value (PPV) 2.5, 95% CI, 1.8 - 3.6; negative predictive value (NPV) 0.46, 95% CI, 0.31 - 0.68], and 53% for Kerning's sign (PPV 3.5, 95% CI, 2.10 - 5.70; NPV 0.56, 95% CI, 0.41 - 0.75) (21). This suggests that meningeal signs during physical examination increase the diagnostic probability of meningitis. However, the absence of meningeal signs cannot be used as isolated data to exclude the diagnosis.
The prevalence of neurological sequelae was relatively low in our study compared to previous studies conducted in the pediatric population (4, 22, 23). For example, Namani et al. developed a retrospective cohort with pediatric patients between one month and 16 years. They presented neurological complications, defined as subdural effusion, recurrent seizures, hemiparesis, intracerebral hemorrhage, cerebritis, facial nerve palsy, hydrocephalus, subdural hematoma, cerebral abscess, subdural empyema, and purulent ventriculitis, with a prevalence of 43% (4). Pelkonen et al. performed a large cohort study in Angola with 403 patients. They reported 25% of severe neurological sequelae at hospital discharge, including blindness, quadriplegia or paresis, hydrocephalus requiring a shunt, and severe psychomotor retardation (22). Factors, such as lack of otorhinolaryngological evaluation of all patients and absence of outpatient follow-up after hospital discharge, could justify the data, since mild neurological sequelae, such as mild neurocognitive developmental delay usually detected only in school time and mild hearing loss, may not have been adequately identified.
Although age was consistently associated with negative outcomes in previous studies and was identified as a possible risk factor for sequelae in the univariate analysis of this cohort, the association was not significant after adjustments. Children diagnosed with S. pneumoniae and H. influenzae type B meningitis were identified with a higher probability of permanent neurological damage. The higher pathogenicity of S. pneumoniae, when compared to other etiological agents, is well established in the literature and explains its association with permanent deficits (8, 23). Theodoridou et al. (14) established S. pneumoniae as a risk factor for sequelae in patients diagnosed with bacterial meningitis, with OR 10.47 (95% CI, 3.14 - 33.82) for developing epilepsy and OR 1.42 (95% CI, 0.36 - 5.60) for severe hearing loss. Correa-Lima et al. (24) demonstrated an association between S. pneumoniae and the development of epileptic frames [OR 4.55 (95% CI, 1.88 - 11.0)]. Also, Wee et al. (25) showed an association between residual sequela after five years of a meningitis episode and identifying H. influenzae type B as the causative agent of infection [OR 29.5 (95% CI, 2 - 429)].
Namani et al. (26) reported that the highest incidence rates of neurological complications, including suppurative complications, were associated with the etiological agents, H. influenzae type B (RR 1.94), and S. pneumoniae (RR 2.57). Although both agents were associated with the presence of sequelae in our study, the etiological agent was not associated with suppurative complications in multivariate analysis.
The association between convulsive crises at admission and the prognosis of patients with bacterial meningitis has already been widely explored. Several authors associated the presence of convulsive crises, either on admission or later, with increased suppurative complications, risk of death, or neurological sequelae (4, 9-12, 22). Considering that convulsive crises are more prevalent in patients infected by S. pneumoniae, the worst clinical outcome could be associated secondarily with the etiological agent known to present the greatest lethality among the most prevalent agents. It is also associated with a higher prevalence of neurological complications (8, 11, 12, 24). However, after multivariate analysis performed in the current study, S. pneumoniae was identified as a risk factor for suppurative complications, but not for neurologic disorder.
The lethality of 12.4% was compatible with the national and state data and with studies on a population similar to that assessed in the cohort (6, 7, 15). When the main mechanism associated with death was evaluated, a discrete predominance of hemodynamic failure was observed to be associated with sepsis. Previous studies have associated hemodynamic severity as an early death mechanism in about 2/3 of pediatric patients with bacterial meningitis (10, 27). This correlation reinforces the association between this cohort and clinical severity during admission and death.
Despite the association between death and treatment with ceftriaxone, the data must be evaluated with caution for treatment with penicillin. The incidence of death in the group of patients treated with penicillin was very low, which may have influenced the analysis. Furthermore, considering the species' resistance mechanism and the sensitivity profile of S. pneumoniae in the studied community, it is unlikely that resistance to ceftriaxone was the explanation for this association (28).
No other studies assessed the prognostic value of gastrointestinal symptoms on bacterial meningitis in the pediatric population. A possible explanation for the association would be that the symptoms of diarrhea or abdominal distension act as confounding factors in the initial clinical approach. Considering that acute gastroenteritis could explain symptoms such as drowsiness and irritability, secondary to a dehydration condition, the approach of a possible abdominal focus would delay the suspected diagnosis of meningitis and its clinical approach. The delay in the clinical approach with late-onset of antibiotic therapy is well established as an independent risk factor for prognosis in bacterial meningitis (3, 9-11, 22, 29, 30).
Despite the absence of studies evaluating the importance of gastrointestinal symptoms as prognostic factors in bacterial meningitis, Campbell et al. (31) presented a series of cases of meningococcal disease by serogroup W. In these cases, the primary gastrointestinal manifestations were related to high mortality and were associated with findings of intestinal necrosis in necropsy. Interestingly, this atypical presentation was also reported in the Chilean epidemic of 2012, where 24% of meningococcal diseases by serogroup W were diagnosed primarily as gastroenteritis, with an approximate lethality of 57% (32). However, gastrointestinal symptoms did not show a statistically relevant association with any etiologic agent in the current cohort. Moreover, among patients with N. meningitidis infection, none of the serogroups could be associated with these symptoms (P = 0.476). This lack of statistically significant correlation could have been influenced by the high percentage of N. meningitidis without serogroup identification (34%).
This study presents a few limitations that must be considered when interpreting the results. The time elapsed between onset of symptoms and the institution of antimicrobial treatment could influence the severity of the patients’ initial clinical presentation and, consequently, their clinical outcome. This data was not evaluated by the authors.
A reduction in meningitis by N. meningitidis and S. pneumoniae was observed after development of conjugate vaccines and emergence of W and Y serogroups among the meningococcal cases. However, a more robust study should be developed on the subject, since it was not possible to access data on the patients' vaccination status to identify whether cases of meningitis by etiological agents that should be covered by the vaccination calendar occur in age groups not covered by the vaccine, due to low adherence of the population to the NIP or individual patient issues (e.g., possible immunodeficiency).
5.1. Conclusions
Despite the limitations, this study fulfilled its main objectives. It showed that S. pneumoniae and H. influenzae type B were associated with sequelae diagnosis during hospital discharge, seizures were considered a risk factor for suppurative complications, and gastrointestinal symptoms or signs of clinical severity were associated with death.
The current study corroborates with the literature and encourages suspicion in the face of febrile conditions with non-specific symptoms, especially when accompanied by convulsive crises or irritability, to avoid misdiagnosis of meningitis. In older children, the classic triad of fever, headache, and vomiting may assist the clinical approach, and meningeal signs increase the diagnostic probability.