1. Background
Ventilator-associated pneumonia (VAP) is a serious problem in intensive care units (ICU), which complicates the underlying disease, increases the length of hospitalization, and imposes an additional burden on the healthcare system. VAP increases the period of mechanical ventilation and hospitalization in the ICU by four to six days and is associated with a mortality rate of 16 to 78%. The risk of VAP increases with an increase in the duration of mechanical ventilation. The most common pathogens responsible for VAP include Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella, and Acinetobacter, which are highly drug-resistant. Therefore, 50% of Staphylococcus aureus species are resistant to methicillin, and 25 to 30% of Pseudomonas and Klebsiella species are resistant to ceftazidime and cefepime. Meanwhile, 60% of cultured Acinetobacter species show resistance to carbapenem (1).
Acinetobacter is a Gram-negative bacterium and a major cause of nosocomial infections, including VAP in severely ill patients with a compromised immune system and immobility. This bacterium is resistant to most classes of antibiotics and is treated with colistin, which is used for multidrug resistance (MDR) (1). The classic signs and symptoms of VAP include fever, leukocytosis, purulent discharge, and poor oxygenation. Cultivation of bronchoalveolar lavage (BAL) secretions has shown a sensitivity of 51%, a specificity of 77%, and a positive predictive value (PPV) of 67%. A variety of imaging techniques, depending on the clinical suspicion, are also helpful. Besides, there are several scoring methods to improve the diagnosis, the most practical of which is the clinical pulmonary infection score (CPIS). According to previous studies on autopsy specimens, the sensitivity and specificity of this criterion were reported to be 46 and 60%, respectively (1, 2).
In addition to standard intravenous (IV) therapy, inhaled antibiotics have been used due to their greater pulmonary absorption and lower systemic toxicity. These benefits are especially important for nephrotoxic drugs with low penetration into the lungs, such as colistin and aminoglycosides. Inhaled antibiotics have been studied both as adjunctive therapy and as alternatives to IV drugs. Although there are few clinical trials in this field, evidence is very limited, especially regarding the use of inhaled antibiotics as an alternative to IV drugs. In studies that used drugs as an adjunctive therapy, the results indicated an increase in microbiological clearance without affecting the length of hospital stay, ventilator dependence, or mortality (3-7).
2. Objectives
Considering the contradictory results regarding the effectiveness of inhaled antibiotics in the treatment of VAP (3, 8-11) and the safety of this method, besides the lack of similar studies in Iran, the present study aimed to compare the effects of inhaled colistin and inhaled amikacin-fosfomycin combination in the treatment of XDR Acinetobacter baumannii-associated VAP. This clinical trial is the first study to evaluate the effect of inhaled fosfomycin on VAP in Iran.
3. Methods
3.1. Trial Design and Participants
This randomized, double-blind clinical trial was conducted in Al-Zahra Hospital, Isfahan, Iran, in 2020. It was approved by the Ethics Committee of Isfahan University of Medical Sciences (grant No.: IR.MUI.MED.REC.1398.139) and registered in the Iranian Registry of Clinical Trials (IRCTID: IRCT20171230038142N13). Written informed consent was obtained from all subjects before the study. The inclusion criteria were hospitalization in the ICU; diagnosis of VAP caused by XDR Acinetobacter (CPIS > 6); and age range of 20 - 65 years. On the other hand, patients were excluded from the study if they had any other concomitant infectious diseases, prescription of additional antibiotics for another reason, or renal dysfunction.
The extensively drug-resistant (XDR) Acinetobacter baumannii was defined according to a study by Magiorakos et al. (1). The CPIS was used to diagnose VAP. According to this criterion, six items of temperature, white blood cell count, amount and type of pulmonary secretions, staining and culture results, PaO2/FiO2 ratio, and radiographic results were examined, with each item scored from 0 to 2. A final score > 6 was considered as the diagnostic threshold. Besides, sputum sampling for culture studies was performed by the tracheal aspiration method with a sterile suction catheter as the least invasive method.
3.2. Treatment Protocol
After the diagnosis of VAP, the patients were empirically treated with 750 mg of levofloxacin daily, 1 g of vancomycin every 12 hours, and 1 g of IV meropenem every eight hours. Once the culture result was determined, patients with XDR Acinetobacter were randomly classified into two groups. In addition to treatment with IV meropenem (2 g every 8 hours via slow infusion) plus IV colistin (9 MIU stat then 4.5 MIU every 12 hours), the first group received inhaled colistin (1 MIU every 8 hours), while the second group received inhaled amikacin (300 mg every 12 hours) plus inhaled fosfomycin (2 cc every 12 hours).
3.2.1. Fosfomycin Preparation
To prepare the fosfomycin inhalation solution, fosfomycin disodium salt was used. Overall, fosfomycin disodium shows higher solubility in water and is more suitable for nebulization in the inhalation dosage form. In this study, a drug concentration of 4% w/v was used to prepare the fosfomycin solution; to prepare an appropriate dose of the drug, 2 mL of the solution was equivalent to 80 mg of the drug. A specific amount of the solution (2 mL) was added to a jet nebulizer chamber and then applied. For nebulization of amikacin, 300 mg of injectable powder was dissolved in 3 mL of distilled sterile water. It should be noted that the filtration method with 100-nm filters was used to prepare the sterile fosfomycin solution. The pH of the solution was also adjusted by a small amount of hydrochloric acid (HCL). The prepared solution was diluted with the nebulizer steam, and tonicity of the solution was adjusted and maintained for body fluids to prevent irritation when inhaled. Also, to prevent the possibility of chemical incompatibility of soluble fosfomycin with amikacin, both drugs were used separately in the nebulizer.
3.2.2. Outcome Assessments
The treatment criteria were as follows: termination of fever, reduction of lung secretion, or radiographic changes. Moreover, the mortality rate, duration of treatment until recovery, and withdrawal from VAP during the intervention were determined and compared between the two groups.
3.2.3. Statistical Analysis
The convenience sampling method was used in this study, and the sample size was measured to be 30 per group, based on the sample size estimation formula to compare the ratios (95% CI; 80% power), considering a 10% prevalence rate of non-response to treatment with colistin (20) and the least significant difference of 0.2 between the groups. The random allocation of patients to the groups was performed using the random allocation software. The obtained data were finally entered into SPSS version 26 and analyzed by Chi-square test, t-test, paired t-test, and repeated measures analysis of variance (ANOVA).
4. Results
In this study, 60 patients with XDR Acinetobacter VAP were examined in two groups of 30 patients, receiving inhaled colistin (group 1) and inhaled amikacin plus inhaled fosfomycin (group 2). Both groups also received high-dose meropenem plus IV colistin. As shown in Table 1, the two groups were not significantly different in terms of age and sex distribution, cause of ICU admission, underlying disease, recent antibiotic use, and history of ICU admission. Also, there was no significant difference between the two groups in terms of the time interval between ICU admission and VAP diagnosis, duration of ventilation until VAP, co-infection with other infectious diseases, and symptoms of sepsis associated with VAP. None of the patients had a history of colistin use. With respect to the underlying disease, 18 (30%) patients had diabetes mellitus, 19 (31.7%) had heart disease, 5 (8.3%) had a history of dialysis, 1 (1.7%) had liver disease, 3 (5%) had a malignancy, and 21 (35%) had other diseases; however, the difference was not significant between the two groups.
| Variables | Groups | P-Value | |
|---|---|---|---|
| Colistin | Amikacin/Fosfomycin | ||
| Age (y) | 16.6 ± 65.4 | 20 ± 57 | 0.09 |
| Sex | 0.18 | ||
| Male | 17 (56.7) | 22 (73.3) | |
| Female | 13 (43.3) | 8 (26.7) | |
| Reason for ICU admission | 0.66 | ||
| Reduced consciousness | 14 (46.7) | 16 (53.3) | |
| Multiple trauma | 2 (6.7) | 5 (16.7) | |
| Respiratory distress | 6 (20) | 6 (20) | |
| Cerebral hemorrhage | 3 (10) | 2 (6.67) | |
| Surgery | 3 (10) | 1 (3.3) | |
| Internal bleeding | 2 (6.7) | 0 (0) | |
| Having an underlying disease | 26 (86.7) | 23 (76.7) | 0.32 |
| Recent use of antibiotics | 25 (83.3) | 26 (86.7) | 0.72 |
| Previous use of carbapenem | 14 (46.7) | 16 (53.3) | 0.61 |
| Previous admission to ICU | 7 (23.3) | 10 (33.3) | 0.39 |
| Time interval between ICU admission and VAP diagnosis (days) | 3.98 ± 16.97 | 9.29 ± 15.31 | 0.71 |
| Duration of mechanical ventilation before VAP | 4.28 ± 18.66 | 9.72 ± 15.59 | 0.51 |
| Concomitant infectious diseases | 5 (16.7) | 1 (3.3) | 0.195 |
| Symptoms of sepsis associated with VAP | 1 | ||
| None | 1 (2.6) | 0 (0) | |
| Sepsis | 17 (56.7) | 17 (56.7) | |
| Severe sepsis | 13 (43.3) | 13 (43.3) | |
The Demographic Characteristics and Clinical History of the Patients in the Two Groups
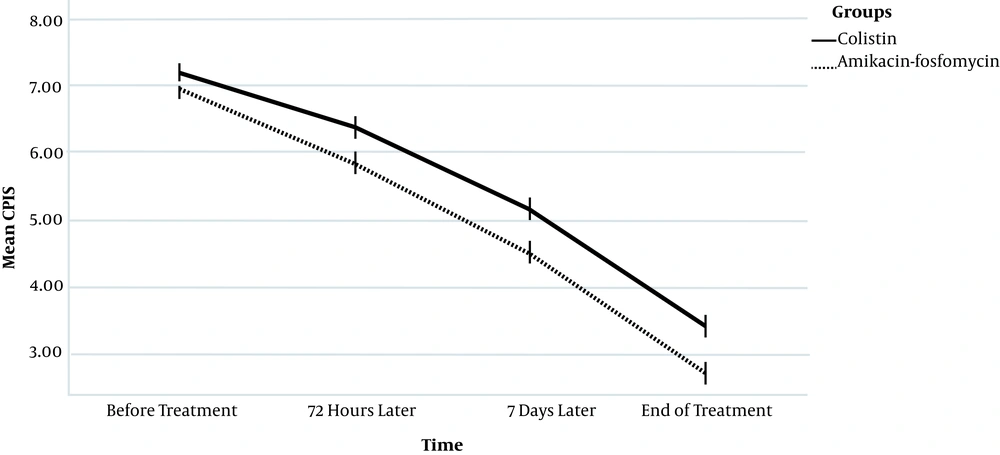
As shown in Table 2, the mean CPIS score at the beginning of treatment was not significantly different between the two groups. However, at 72 hours and seven days after treatment and also at the end of treatment, the mean score of the amikacin-fosfomycin group was significantly lower than the other group. Also, according to the intra-group studies, the CPIS score was significantly reduced in both groups (P < 0.001). According to the inter-group analysis, the mean CPIS changes were significantly different between the groups; in the amikacin-fosfomycin group, a greater decrease in the CPIS score was observed as compared to the colistin group (P = 0.007; Figure 1).
| Time | Groups | P-Value | |
|---|---|---|---|
| Colistin | Amikacin/Fosfomycin | ||
| Upon diagnosis | 0.7 ± 7.17 | 0.83 ± 7.07 | 0.62 |
| After 72 hours | 0.77 ± 6.43 | 0.91 ± 5.93 | 0.025 |
| After seven days | 1.38 ± 5.29 | 1.06 ± 4.55 | 0.028 |
| End of treatment | 0.78 ± 3.42 | 0.81 ± 2.71 | 0.003 |
| P-value | < 0.001 | < 0.001 | 0.007 |
The Mean ± SD CPIS During the Intervention in the Two Groups
Before treatment, the mean erythrocyte sedimentation rates (ESRs) in the colistin and amikacin-fosfomycin groups were 51.57 ± 24.8 and 48.17 ± 27.3, respectively (P = 0.051). The mean C-reactive protein (CRP) level at the beginning of treatment was 21.83 ± 71.69 and 28.4 ± 72.77 in the colistin and amikacin-fosfomycin groups, respectively, and no significant difference was observed between these groups (P = 0.87). Moreover, as shown in Table 3, the serum levels of procalcitonin before and after treatment did not differ significantly between the two groups, while the difference was significant between the beginning and end of treatment in both groups. On the other hand, the trend of changes in procalcitonin was not significantly different between the two groups (P = 0.87). The creatinine and blood urea levels were not significantly different between the two groups during the study (P = 0.37 and P = 0.61, respectively).
| Variables/Time | Groups | P-Value | |
|---|---|---|---|
| Colistin | Amikacin/Fosfomycin | ||
| Procalcitonin | |||
| Beginning of treatment | 0.77 ± 4.16 | 0.89 ± 5.59 | 0.23 |
| End of treatment | 0.7 ± 1.87 | 0.23 ± 1.06 | 0.27 |
| P-value | 0.049 | 0.021 | 0.87 |
| Creatinine | |||
| Upon diagnosis | 0.81 ± 1.35 | 1.24 ± 1.49 | 0.62 |
| After 72 hours | 1.25 ± 1.64 | 1.33 ± 1.54 | 0.76 |
| After seven days | 1.16 ± 1.79 | 1.15 ± 1.52 | 0.37 |
| End of treatment | 2.67 ± 2.26 | 0.95 ± 1.42 | 0.13 |
| P-value | 0.22 | 0.46 | 0.31 |
| Serum urea | |||
| Upon diagnosis | 16.68 ± 28.34 | 21.93 ± 25.73 | 0.61 |
| After 72 hours | 18.14 ± 30.9 | 26.49 ± 27.18 | 0.53 |
| After seven days | 16.74 ± 31.27 | 20.61 ± 26.93 | 0.38 |
| End of treatment | 13.98 ± 24.88 | 15.66 ± 24.41 | 0.91 |
| P-value | 0.16 | 0.64 | 0.61 |
The Mean ± SD of Serum Procalcitonin, Urea, and Creatinine Levels in the Two Groups
The blood culture results were negative at seven days after the onset of treatment in 10 (33.3%) patients in the colistin group and in 28 (93.3%) patients in the amikacin-fosfomycin group (P = 0.001). The mean duration of treatment was 13.61 ± 3.18 and 3.18 ± 10.41 days in the colistin and amikacin-fosfomycin groups, respectively; the mean duration of treatment in the amikacin-fosfomycin group was significantly lower than the colistin group (P < 0.001).
There were 6 (20%) cases of non-response to treatment in the colistin group and 5 (16.7%) cases in the amikacin-fosfomycin group (P = 0.74). Among patients who did not respond to treatment, 2 (6.7%) died in the amikacin-fosfomycin group. Besides, the treatment plan changed for 6 (20%) patients in the colistin group and for 3 (10%) patients in the amikacin-fosfomycin group (P = 0.31). In terms of drug side effects, 11 (36.7%) patients in the colistin group and 5 (16.7%) patients in the amikacin-fosfomycin group developed the symptoms of nephrotoxicity (P = 0.08).
5. Discussion
Acinetobacter baumanii is one of the main causes of VAP in ICUs. Because of its high prevalence and the use of various antibiotics, we observed MDR in this bacterium. Colistin is a drug currently used to treat VAP caused by Acinetobacter; however, this treatment is not optimal, and the mortality rate, as well as the length of ICU stay, is high. Although some studies have shown that nebulization of some antibiotics, along with the IV use of broad-spectrum antibiotics, such as colistin, can increase and accelerate the recovery of VAP, the scope of studies in this field is limited. Therefore, the present study aimed to compare the effects of inhaled colistin and a combination of inhaled amikacin-fosfomycin in the treatment of VAP caused by XDR Acinetobacter.
In the present study, the two groups treated with colistin and amikacin/fosfomycin did not differ significantly in terms of demographic and baseline characteristics, such as age and sex distribution, underlying disease, history of hospitalization, cause of ICU admission, and serological findings at the beginning of the study. No distortion of the abovementioned factors was observed in the treatment results. The findings of our study showed that both nebulization methods of colistin and amikacin-fosfomycin reduced the duration of treatment and also increased the recovery rate of patients with VAP caused by Acinetobacter. Simultaneously, in patients treated with nebulized amikacin-fosfomycin, the recovery rate was higher, and the CPIS score further decreased. In this regard, a study by Kollef et al. on 143 patients with VAP, induced by gram-negative bacteria, compared the effect of 120 mg of fosfomycin plus 300 mg of inhaled amikacin as an adjunctive therapy to the standard IV regimen versus inhaled saline (placebo). The findings showed a significant reduction in the number of positive tracheal cultures in the group of inhaled antibiotics (3), which is consistent with our results.
In a review study by Wood et al., the effects of inhaled antibiotics on hospital-acquired pneumonia (HAP)/VAP treatment were assessed during 2010 - 2017. In previous clinical trials, the used antibiotics mostly included colistin and aminoglycosides, and the most common pathogens were Pseudomonas and Acinetobacter. There are contradictory results about the clinical effects of inhaled antibiotics. However, almost half of previous studies reported better clinical outcomes, and the use of these antibiotics did not have any severe side effects (4). In this regard, Montgomery A. Bruce et al. (2014) prescribed different doses of inhaled fosfomycin and amikacin to patients with VAP using an inline nebulizer. They found that 80-mg fosfomycin and two million units of amikacin were more effective without causing any clinical complications or reducing oxygen saturation (5).
Moreover, a meta-analysis of 12 studies, including six clinical trials, showed that inhaled antibiotics produced better clinical outcomes. In these 12 studies, the most common pathogens were Acinetobacter baumannii and Klebsiella pneumonia, and the most common inhaled antibiotics were colistin (in nine studies) and aminoglycosides (in seven studies, including three cases of tobramycin) (6). Besides, in a retrospective cohort study on the effects of inhaled adjunctive colistin and tobramycin in the treatment of 93 patients with VAP, caused by Pseudomonas and Acinetobacter, higher survival rates were found despite MDR (7).
Moreover, Lu et al. treated 165 patients with VAP, caused by P. aeruginosa or A. baumannii, using beta-lactam and aminoglycoside or quinolone-susceptible strains for 14 days with IV antibiotics. Patients with MDR were treated with inhaled colistin. After 14 days, the clinical response, mortality, and nephrotoxicity were similar between Pseudomonas and Acinetobacter (8). In another study, the effects of ceftazidime and amikacin on the clinical outcomes of patients with VAP due to P. aeruginosa were similar in the inhaled and IV groups (9). Also, in a study by Hallal et al., the outcomes of patients with VAP, caused by P. aeruginosa or Acinetobacter, in the inhaled tobramycin group were more favorable than the IV group (10). Besides, the use of inhaled colistin as an adjunctive therapy for patients with HAP (including VAP), induced by Gram-negative resistant strains, improved the outcomes of these patients (11).
The results of the majority of studies on the use of inhaled antibiotics are in line with the results of our study and indicate the positive effects of nebulized antibiotics, especially amikacin/fosfomycin in accelerating the treatment process and improving the outcomes of patients. This is probably related to the faster and higher dose of antibiotics, reaching the site of infection (lung tissue). Besides its direct effect on infection, fosfomycin may be also helpful in increasing the effectiveness of other antibiotics, including colistin and amikacin (3).
The present study had some limitations, including the relatively high incidence of nephrotoxicity symptoms in both groups, although it was lower than the rates of previously mentioned studies (4, 5, 8). The nephrotoxicity symptoms could probably be attributed to the use of IV drugs, such as colistin. Also, considering the drug pharmacokinetics and the lack of inhaled drugs in the circulatory system, they are less likely to cause kidney poisoning. Finally, the small sample size is another limitation of this study. Therefore, designing similar studies with a larger sample size can lead to clearer results.
5.1. Conclusion
The findings of the present study showed that the use of nebulized amikacin-fosfomycin can lead to improvements and reduce the treatment duration in patients with VAP, caused by XDR Acinetobacter. However, large-scale, appropriately designed, randomized controlled clinical trials are needed to evaluate the efficacy of these therapeutic agents.