1. Background
The first report of coronavirus disease 2019 (COVID-19) in Iran was officially announced on February 19, 2020, from the city of Qom in central Iran (1). Shortly thereafter, cases of infection with the novel coronavirus were reported from all over the country.
Although case fatality rate (CFR) in patients admitted to hospital and its associated clinical factors are some of the most substantial indicators that should be evaluated in COVID-19 pandemic, the complexity, and challenges, particularly in initial weeks of the pandemic, can cause lots of uncertainty. Among these complexities are: The rate of hospital and intensive care unit (ICU) admission (the former as the denominator of the CFR and the latter as one of the main courses of treatment), the criteria for hospital and ICU admissions, sensitivity and specificity of the diagnostic tests (including real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) and computed tomography (CT scan), low access to and experience with diagnostic tests, and last but not least the treatment regimens administered. Furthermore, such factors as changing the definitions and criteria, inadequate hospital dataset standards, and incomplete data can dramatically change CFR assessment.
In the first wave of COVID-19 outbreak, when debates on the effect of anti-viral or any other drug regimen are still challenging, effective ICU services for the management of severe cases is crucial, although deficiencies may occur due to the number of cases or inappropriate allocation protocols. This situation provided a unique opportunity to not only assess the clinical pattern and outcome of the disease in Qazvin province, Iran but also, evaluate the performance of 12 hospitals throughout the province during the critical first three weeks of emergence the outbreak.
The results of such an assessment can further be employed as a basis for more precise hospital and ICU admission criteria aimed at saving more lives in the subsequent critical phases of the epidemic.
2. Objectives
Little data has been documented about the course and outcome of the affected patients in the country since the emergence of the COVID-19 epidemic in Iran. In this retrospective cohort study, we aimed to investigate the clinical pattern and outcome of patients with a primary diagnosis of COVID-19 but different rRT-PCR test results admitted to the hospitals and ICUs of Qazvin province Iran, from February 20, 2020, to March 11, 2020.
3. Methods
In this retrospective cohort study, patients with a primary diagnosis of COVID-19 (see definition) admitted to all 12 hospitals across Qazvin province, Iran, from February 20, 2020, to March 11, 2020, were included and followed up until March 27, 2020.
Upon the outbreak, the Qazvin University of Medical Sciences, as the provincial health governing body, provided an electronic data entry platform to collect epidemiologic and clinical data related to patients with COVID-19 and mandated all involved hospitals within the province to employ the database. Data were collected from patients’ medical records and entered into the electronic database during the patients’ admission period. This acquisition of data included epidemiological and clinical data, patients’ exposure history, as well as the results of their COVID-19 rRT-PCR test and clinical outcome (i.e., discharge or death). After the discharge of patients, they were followed up by phone until March 27, 2020 to confirm the outcome.
This study was approved by the Research Ethics Committee of Qazvin University of Medical Sciences (Code: IR.QUMS.REC.1399.007). An informed consent was obtained from the patients upon admission.
3.1. Inclusion Criteria
Patients were admitted with a primary diagnosis of COVID-19 based on the INIGCDT (2). This edition, published on February 24, 2020, limited the indications for rRT-PCR testing to detect SARS-CoV-2 to those admitted with severe respiratory signs and symptoms or admitted patients with fever whose chest imaging revealed pulmonary infiltration (3). According to the guidelines of the INIGCDT, a primary diagnosis of COVID-19 is characterized by symptoms of fever, cough, or myalgia (referred herein as minor clinical criteria), coupled with either A) respiratory distress, low pulse oximetry reading (SpO2 ≤ 93%), respiratory rate (RR) of > 30 (herein considered as major clinical criteria), or decreased level of consciousness; or B) patients being among high-risk groups who have an underlying medical condition along with suggestive chest X-ray or CT scan changes for COVID-19 (see below). Further laboratory and chest imaging studies were performed during the patients’ admission course.
A definitive diagnosis of COVID-19 was made using rRT-PCR to detect SARS-CoV-2 in patients’ respiratory secretions. The test became available through the Pasteur Institute of Iran in early February 2020. Throat swab (nasopharyngeal and oropharyngeal) for initial diagnosis and lower respiratory secretion specimens (by induced sputum sample or in intubated patients) for definitive diagnosis were obtained from patients and sent for SARS-CoV-2 PCR test during their admission course. However, due to a serious shortage of these tests in Iran at the time, patients were not reexamined.
Fever was defined as an oral temperature of > 37.8°C. Shortness of breath and decreased level of consciousness were defined upon the triage physician’s clinical judgement. Underlying medical conditions that justified patients’ admission, as proposed in the INIGCDT, included a history of cardiovascular disease, pulmonary disease, diabetes, hypertension, cancer, human immunodeficiency virus (HIV) infection, and organ transplantation. Chest X-ray or CT scan changes suggestive of COVID-19 consisted of bilateral patchy infiltration with rapid progression toward ground glass opacity (GGO) (2, 3).
Patients were admitted to ICUs if they had persistent hypoxemia, decreased level of consciousness, hemodynamic instability, or hypercapnia.
Patients were discharged from the hospital if they had no fever for more than 48 - 72 hours, had an SpO2 of > 93% while breathing ambient air, had improved clinical symptoms and signs, and showed remarkable improvement in their serial chest imaging.
For comparison, patients were categorized into four groups including: (1) survivors outside the ICU; (2) survivors inside the ICU; (3) non-survivors outside the ICU; and (4) non-survivors inside the ICU.
3.2. Patients’ Follow-up
The patients who were admitted during the third week of the study period (5 - 7 March, 2020) were actively followed up using phone interviews on March 27. Among them, 436 patients (response rate = 76.7%) were reached.
3.3. Statistical Analysis
Descriptive analyses were carried out using the median (IQR). Patients were categorized based on their week of admission, outcome inside and outside the ICU, and their rRT-PCR testing status. The CFR was assessed for each group. The chi-square test was employed to assess differences in study variables among the different categories of patients.
A multiple logistic regression model was applied to identify the factors associated with death in COVID-19 patients. The odds ratio (OR) and 95% confidence interval (CI) were determined for each contributing factor. Then, patients were categorized into six groups based on receiving the intensive care services and rRT-PCR test status (positive, negative, or no test). Also, multilevel logistic regression was used to compare the odds of surviving in each group against the reference group (PCR negative patients not-received ICU) to show if the rational allocation of ICU occurred while its capacity is limited. The constructed model was adjusted for sex, age, Charlson Comorbidity Index (CCI), calendar time, oxygen saturation, and type of hospital. The difference between compared groups was summarized in terms of OR. It also allowed us to calculate the mortality rate for each PCR/ICU group adjusted for factors associated with in-hospital mortality using the ‘margins’ and ‘predict’ commands in Stata software version 14.1.
Statistical analysis was performed using the Stata statistical software package (StataCorp. 2014. Stata Statistical Software: Release 14.1, College Station, TX: StataCorp LP). A P value < 0.05 was considered as significant.
4. Results
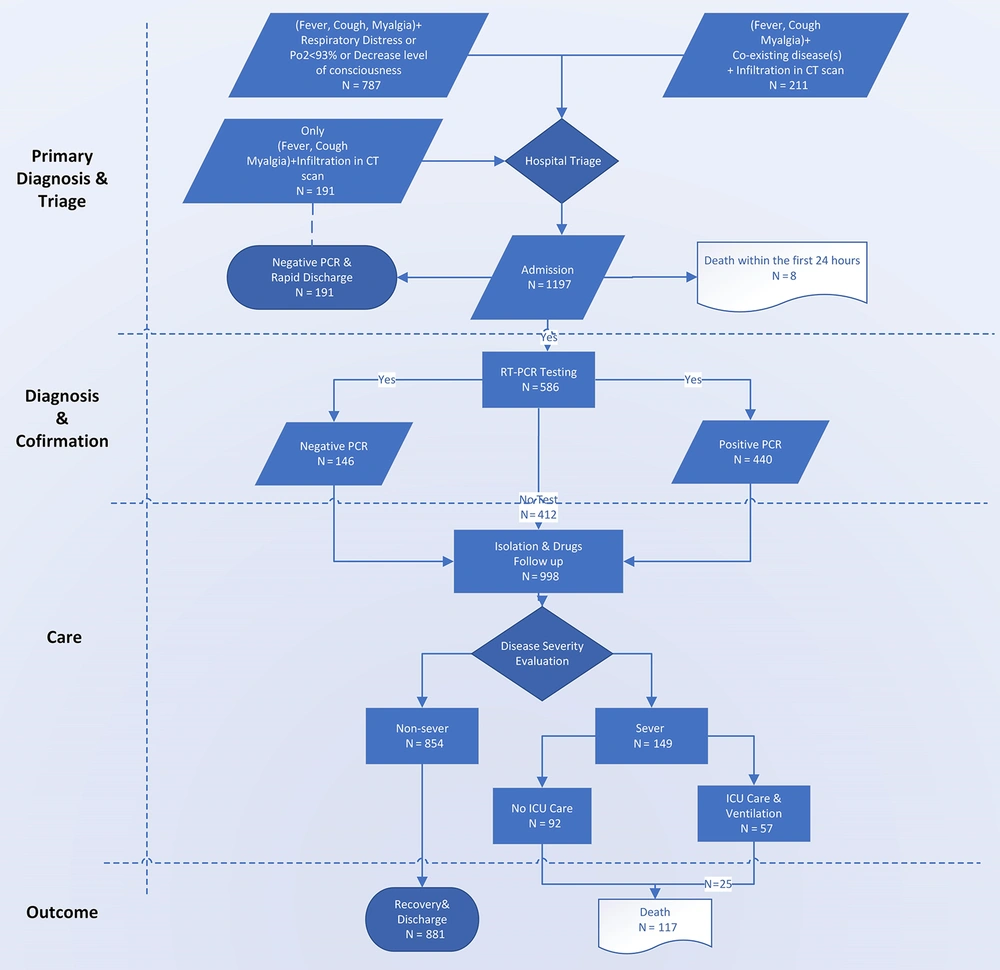
The data of 1,197 patients admitted to hospitals in Qazvin province were entered into the related electronic database from February 20 to March 11, 2020. Among these patients, 191 patients who were PCR-negative and were admitted to non-COVID-19 wards were excluded from the analysis; however, their clinical course was followed up, as with all patients. The CFR among the latter group was 3.1%, with none of them being admitted to ICUs. Furthermore, eight patients whose death occurred within the first 24 hours of their admission were excluded from the study (Figure 1).
A total of 998 patients (57% male) with a median age of 54 (IQR 25 - 75 = 25) years were analyzed. Among them, 558 patients were tested for COVID-19, with 412 testing positive and 146 testing negative. ICU care was provided for 52% of patients, while the remaining 48% were isolated in the designated wards where they received low-flow oxygen and medical therapy (Table 1).
| Variables | Week 1 (N = 116) | Week 2 (N = 399) | Week 3 (N = 483) | Total (N = 998) |
|---|---|---|---|---|
| Sex | ||||
| Female | 57 (49.14) | 168 (42.11) | 204 (42.24) | 429 (42.99) |
| Male | 59 (50.86) | 231 (57.89) | 279 (57.76) | 569 (57.01) |
| Age group (y) | ||||
| < 50 | 55 (47.41) | 167 (41.85) | 180 (37.27) | 420 (40.28) |
| 51 - 60 | 21 (18.10) | 86 (21.55) | 85 (17.60) | 192 (19.24) |
| 61 - 70 | 17 (14.66) | 74 (18.55) | 96 (19.88) | 187 (18.74) |
| ≥ 70 | 23 (19.83) | 72 (18.05) | 122 (25.26) | 217 (21.74) |
| Admission criteria | ||||
| Minor clinical criteria b plus at least one major criteria c | 92 (79.31) | 317 (79.45) | 378 (78.26) | 787 (78.86) |
| Co-existing disorders OR immunodeficiency d | 24 (20.69) | 82 (20.55) | 105 (21.74) | 211 (21.14) |
| rRT-PCR test status | ||||
| Positive | 33 (28.45) | 200 (50.13) | 207 (42.86) | 440 (44.09) |
| Negative | 20 (17.24) | 49 (12.28) | 77 (15.94) | 146 (14.63) |
| Not tested | 63 (54.31) | 150 (37.59) | 199 (41.20) | 412 (41.28) |
| ICU admission | ||||
| No | 108 (93.10) | 377 (94.49) | 461 (95.45) | 946 (94.70) |
| Yes | 8 (6.90) | 22 (5.51) | 22 (4.55) | 52 (5.21) |
| Co-existing disorders | ||||
| Two or more comorbidities | 12 (12.12) | 32 (9.94) | 36 (9.00) | 80 (9.74) |
| Cardiovascular diseases | 16 (13.79) | 41 (10.28) | 53 (10.97) | 110 (11.02) |
| Diabetes | 13 (11.21) | 43 (10.78) | 49 (10.14) | 105 (10.52) |
| Hypertension | 4 (3.45) | 30 (7.52) | 23 (4.76) | 57 (5.71) |
| Chronic pulmonary diseases | 4 (3.45) | 17 (4.26) | 9 (1.86) | 30 (3.01) |
| Immunodeficiency d | 1 (0.86) | 6 (1.50) | 9 (1.86) | 16 (1.60) |
| Chronic renal diseases | 1 (0.86) | 4 (1.00) | 5 (1.04) | 10 (1.00) |
| Total | 30 (25.86) | 111 (27.82) | 124 (25.67) | 265 (26.55) |
| Hospital type | ||||
| Teaching | 92 (79.31) | 317 (79.45) | 360 (74.53) | 769 (77.05) |
| High volume non-teaching | 17 (14.66) | 78 (19.55) | 117 (24.22) | 212 (21.24) |
| Low volume e non-teaching | 7 (6.03) | 4 (1.00) | 6 (1.24) | 17 (1.70) |
| Outcome | ||||
| Recovery | 104 (89.66) | 352 (88.22) | 425 (87.99) | 881 (88.28) |
| Death | 12 (10.34) | 47 (11.78) | 58 (12.01) | 117 (11.72) |
Basic Characteristics of the Study Participants a
Comparing the study participants’ characteristics between the four groups of survivors and non-survivors outside and inside the ICU, no sex difference in CFR was found (P = 0.674). Among the non-survivors, except for those aged < 50 years, death rates were higher in patients outside the ICU than inside; this difference was more prominent among patients aged > 70 years (23.04 vs. 4.15%, respectively). Notably, in the rRT-PCR test-positive group, a 20.68% CFR was observed (Table 2).
| Variables | Total (N = 998) | Non-survivors; N = 117 (11.7%) | Survivors; N = 881 (88.3%) | P Value | ||
|---|---|---|---|---|---|---|
| Outside ICU (N = 92) | Inside ICU (N = 25) | Outside ICU (N = 854 | Inside ICU (N = 27) | |||
| Sex | ||||||
| Female | 429 (42.99) | 40 (9.32) | 8 (1.87) | 368 (85.78) | 13 (3.03) | |
| Male | 569 (57.01) | 52 (9.14) | 17 (2.99) | 486 (85.41) | 14 (2.46) | 0.674 |
| Age group (y) | ||||||
| < 50 | 402 (40.28) | 17 (4.05) | 7 (6.67) | 372 (88.57) | 6 (1.43) | |
| 51 - 60 | 192 (19.24) | 9 (4.69) | 3 (1.56) | 174 (90.63) | 6 (3.13) | |
| 61 - 70 | 187 (18.74) | 16 (8.56) | 6 (3.21) | 160 (85.56) | 5 (2.67) | |
| ≥ 70 | 217 (21.74) | 50 (23.04) | 9 (4.15) | 148 (68.20) | 10 (4.61) | < 0.001 |
| RT-PCR test status | ||||||
| Positive | 440 (44.09) | 74 (16.82) | 17 (3.86) | 344 (78.18) | 5 (1.14) | |
| Negative | 146 (14.63) | 6 (4.11) | 5 (3.42) | 123 (84.25) | 12 (8.22) | |
| Not tested | 412 (41.28) | 12 (2.91) | 3 (0.73) | 387 (93.93) | 10 (2.43) | < 0.001 |
| Symptoms | ||||||
| Fever | 545 (54.61) | 50 (9.17) | 8 (1.47) | 476 (87.34) | 11 (2.02) | 0.053 |
| Cough | 666 (66.73) | 63 (9.46) | 15 (2.25) | 583 (87.54) | 8 (1.20) | < 0.001 |
| Myalgia | 301 (30.16) | 22 (7.31) | 3 (1.00) | 271 (90.03) | 5 (1.66) | 0.039 |
| Shortness of breath | 573 (57.42) | 58 (10.12) | 21 (3.67) | 477 (83.25) | 17 (2.97) | 0.022 |
| SpO2 < 93% | 612 (61.32) | 61 (9.97) | 17 (2.78) | 513 (83.82) | 21 (3.43) | 0.166 |
| Unconsciousness | 31 (3.11) | 4 (12.90) | 7 (22.58) | 12 (38.71) | 9 (29.03) | < 0.001 |
| Minor clinical criteria | 185 (18.54) | 13 (7.03) | 3 (1.62) | 166 (89.73) | 3 (1.62) | 0.343 |
| Major clinical criteria | 17 (1.70) | 3 (17.65) | 6 (35.29) | 3 (17.65) | 5 (29.41) | < 0.001 |
| Co-existing disorders | ||||||
| Cardiovascular disease | 110 (11.02) | 20 (18.18) | 6 (5.46) | 81 (73.64) | 3 (2.73) | 0.001 |
| Diabetes | 105 (10.52) | 15 (14.29) | 4 (3.81) | 84 (80.00) | 2 (1.91) | 0.189 |
| ≥ 2 co-morbidities | 80 (9.74) | 15 (18.75) | 5 (6.25) | 55 (68.75) | 5 (6.25) | < 0.001 |
| Hypertension | 57 (5.71) | 7 (12.28) | 5 (8.77) | 41 (71.93) | 4 (7.02) | 0.001 |
| Chronic pulmonary disease | 30 (3.01) | 4 (13.33) | 1 (3.33) | 23 (76.67) | 2 (6.67) | 0.435 |
| Immunodeficiency | 16 (1.60) | 2 (12.50) | 3 (18.75) | 10 (62.50) | 1 (6.25) | < 0.001 |
| Chronic renal disease | 10 (1.00) | 2 (20.00) | 2 (20.00) | 6 (60.00) | 0 (0.00) | 0.002 |
| Week of admission | ||||||
| 1st | 116 | 8 (6.90) | 4 (3.45) | 100 (86.21) | 4 (3.45) | |
| 2nd | 399 | 36 (9.02) | 11 (2.76) | 341 (85.46) | 11 (2.76) | |
| 3rd | 483 | 48 (9.94) | 10 (2.07) | 413 (85.51) | 12 (2.49) | 0.903 |
| Hospital type | ||||||
| Teaching hospital | 769 (77.05) | 71 (9.23) | 22 (2.86) | 658 (85.57) | 18 (2.34) | |
| High volume non-teaching | 212 (21.24) | 20 (9.43) | 2 (0.94) | 184 (86.79) | 6 (2.83) | |
| Low volume non- teaching | 17 (1.70) | 1 (5.88) | 1 (5.88) | 12 (70.59) | 3 (17.65) | 0.005 |
Comparing the study participants based on their PCR test results, it was found that while most tests had been carried out on individuals aged > 70 years than the younger age groups, there was no statistically significant age difference between positive and negative rRT-PCR patients. Moreover, although patients receiving ICU services were tested more than non-ICU admitted patients, positive results were more prevalent in the latter group (Table 3).
| Variables | RT-PCR Test Status | RT-PCR Test Results | ||||
|---|---|---|---|---|---|---|
| Tested (N = 586) | Not Tested (N = 412) | P Value | Test Positive (N = 440) | Test Negative (N = 146) | P Value | |
| Sex | ||||||
| Female | 243 (56.64) | 186 (43.36) | 188 (77.37) | 55 (22.63) | ||
| Male | 343 (60.28) | 226 (39.72) | 0.248 | 252 (73.47) | 91 (26.53) | 0.283 |
| Age group (y) | ||||||
| < 50 | 221 (54.98) | 181 (45.02) | 168 (76.02) | 53 (23.98) | ||
| 51 - 60 | 101 (52.60) | 91 (47.40) | 73 (72.28) | 28 (27.72) | ||
| 61 - 70 | 119 (63.64) | 68 (36.36) | 90 (75.63) | 29 (24.37) | ||
| ≥ 70 | 145 (66.82) | 72 (33.18) | 0.005 | 109 (75.17) | 36 (24.83) | 0.908 |
| ICU admission | ||||||
| Yes | 39 (75.00) | 13 (25.00) | 22 (56.41) | 17 (43.59) | ||
| No | 547 (57.82) | 399 (42.18) | 0.014 | 418 (76.42) | 129 (23.58) | 0.005 |
| Co-existing disorders | ||||||
| No | 405 (55.25) | 328 (44.75) | 310 (76.54) | 95 (23.46) | ||
| Yes | 181 (68.30) | 84 (31.70) | < 0.001 | 130 (71.82) | 51 (28.18) | 0.222 |
| ≥ 2 Comorbidities | 60 (75.00) | 20 (25.00) | 0.001 | 40 (66.67) | 20 (33.33) | 0.096 |
| Cardiovascular diseases | 80 (72.73) | 30 (27.27) | 0.002 | 56 (70.00) | 24 (30.00) | 0.258 |
| Chronic pulmonary diseases | 22 (73.33) | 8 (26.67) | 0.099 | 16 (72.73) | 6 (27.27) | 0.794 |
| Diabetes | 74 (70.48) | 31 (29.52) | 0.010 | 54 (72.97) | 20 (27.03) | 0.653 |
| Hypertension | 38 (66.67) | 19 (33.33) | 0.209 | 26 (68.42) | 12 (31.58) | 0.326 |
| Immunodeficiency | 9 (56.25) | 7 (43.75) | 0.840 | 5 (55.56) | 4 (44.44) | 0.172 |
| Chronic renal diseases | 7 (70.00) | 3 (30.00) | 0.466 | 5 (71.43) | 2 (28.57) | 0.822 |
| Signs and symptoms | ||||||
| Any sign | 551 (57.22) | 412 (42.78) | < 0.001 | 405 (73.50) | 146 (26.50) | < 0.001 |
| Fever | 293 (53.76) | 252 (46.24) | < 0.001 | 224 (76.45) | 69 (23.55) | 0.445 |
| Cough | 376 (55.90) | 295 (44.10) | 0.010 | 288 (77.01) | 86 (22.99) | 0.153 |
| Myalgia | 159 (52.82) | 142 (47.18) | 0.013 | 119 (74.84) | 40 (25.16) | 0.934 |
| Shortness of breath | 301 (52.53) | 272 (47.47) | < 0.001 | 217 (72.09) | 84 (27.91) | 0.085 |
| SpO2 < 93% | 313 (51.14) | 299 (48.86) | < 0.001 | 221 (70.61) | 92 (29.39) | 0.007 |
| Unconsciousness | 22 (68.75) | 10 (31.25) | 0.241 | 11 (50.00) | 11 (50.00) | 0.006 |
| Outcome | ||||||
| Recovery | 484 (54.94) | 397 (45.06) | 349 (72.11) | 135 (27.89) | ||
| Death | 102 (87.18) | 15 (12.82) | < 0.001 | 91 (89.22) | 11 (10.78) | < 0.001 |
| Admission week | ||||||
| 1st | 53 (45.69) | 63 (54.31) | 33 (62.26) | 20 (37.74) | ||
| 2nd | 249 (62.41) | 150 (37.59) | 200 (80.32) | 49 (19.68) | ||
| 3rd | 284 (50.80) | 199 (41.20) | 0.006 | 207 (72.89) | 77 (27.11) | 0.011 |
The CFR among admitted patients in Qazvin province was 11.7% (Table 1); however, the CFR differed between the various groups (Table 4). A multiple logistic regression model including age group, sex, type of hospital (i.e., teaching or non-teaching; high volume or low volume), week of admission, ICU admission, co-existing disorders, and rRT-PCR test results was used to determine the factors associated with death in patients. The results showed that age > 70 years (OR = 5.2; 95% CI = 2.9 - 9.1; P ≤ 0.001), immunodeficiency disorders (OR = 4.3 ; 95% CI = 1.11 - 17.23; P = 0.035), ICU admission (OR = 11.5; 95% CI = 5.5 - 23.9; P ≤ 0.001), and having positive rRT-PCR test results (OR = 5.8; 95% CI = 2.7 - 12.5; P ≤ 0.001) were the main determinants of death in patients. Also, having two comorbidities or more was a weak risk factor for death (OR = 1.8 ; 95% CI = 0.92 - 3.06; P = 0.08). After multi-level regression analysis in the six above-mentioned groups, only the two groups with positive rRT-PCR test results showed significantly higher mortality rates in comparison to the reference group after controlling the effects of confounding variables (OR = 16.8; 95% CI = 5.6 - 49.7; P < 0.001 in ICU admitted group and OR = 4.0; 95% CI = 1.8 - 8.9; P = 0.001 in the non-ICU admitted group).
| Subgroups | Number of Deaths (%) | Odds Ratio (95% CI) | P Value |
|---|---|---|---|
| Sex | |||
| Female | 48 (11.19) | Reference | |
| Male | 69 (12.13) | 1.16 (0.74, 1.81) | 0.511 |
| Age group (y) | |||
| < 50 | 24 (5.97) | Reference | |
| 50 - 59 | 12 (6.25) | 0.97 (0.44, 2.11) | 0.942 |
| 60 - 69 | 22 (11.76) | 1.73 (0.89, 3.35) | 0.101 |
| ≥ 70 | 59 (27.19) | 5.15 (2.91, 9.12) | < 0.001 |
| ICU admission | |||
| No | 92 (9.73) | Reference | |
| Yes | 25 (48.08) | 11.46 (5.53, 23.86) | < 0.001 |
| RT-PCR test status | |||
| Negative | 11 (7.53) | Reference | |
| Positive | 91 (20.68) | 5.75 (2.65, 12.48) | < 0.001 |
| Not tested | 15 (3.64) | 0.74 (0.30, 1.84) | 0.531 |
| Co-existing disorder | |||
| Without co-existing disorder | 67 (9.14) | Reference | |
| All | 50 (18.87) | 1.4 (0.91, 2.34) | 0.110 |
| ≥ 2 Co-disorders | 20 (25.02) | 1.82 (0.92, 3.60) | 0.081 |
| Cardiovascular diseases | 26 (23.64) | 1.57 (0.86, 2.87) | 0.186 |
| Chronic pulmonary diseases | 5 (16.67) | 1.28 (0.43, 3.79) | 0.438 |
| Diabetes | 19 (18.10) | 1.39 (0.73, 2.64) | 0.287 |
| Chronic renal disease | 4 (40.00) | 3.07 (0.55, 17.08) | 0.164 |
| Immunodeficiency | 5 (31.25) | 4.3 (1.11, 17.23) | 0.035 |
| Hypertension | 12 (21.05) | 0.99 (0.44, 2.20) | 0.991 |
| Admission week | |||
| 1st | 12 (10.34) | Reference | |
| 2nd | 47 (11.78) | 0.77 (0.32, 1.75) | 0.536 |
| 3rd | 58 (12.01) | 0.92 (0.41, 2.05) | 0.845 |
| Type of hospital | |||
| Teaching hospitals | 93 (12.09) | Reference | |
| High-volume non-teaching | 22 (10.38) | 0.61 (0.32, 1.11) | 0.140 |
| Low-volume non-teaching | 2 (11.76) | 0.23 (0.03, 1.55) | 0.113 |
5. Discussion
The present study was conducted in the first critical three weeks of the novel coronavirus outbreak in Qazvin province, Iran. The results showed that while CFR was 7.5% among CT-diagnosed COVID-19 patients with typical respiratory symptoms but negative rRT-PCR results, this rate was as high as 20.7% in test-positive patients.
As shown in Table 1, a similar CFR was observed in both males and females. This finding is in line with the reports from Wuhan, China (3). On the other hand, the CFR in patients aged > 70 years and in those who were ICU-admitted was higher compared to the reference group. Nevertheless, we did not find a significant increased death rate among COVID-19 patients with coexisting diseases. The exception, however, was in those with immunodeficiency disorders, in whom the CFR was about four times that of the reference group (Table 3). In fact, the regression model showed that age affected death both independently and through a “corridor” of comorbidities. On the other hand, the adverse effects of major comorbidities were observed only in association with age, i.e., major comorbidities had no independent role in the CFR in this cohort of patients. In contrast, Guan et al. reported that, among hospitalized PCR-tested patients, the composite endpoint, including admission to ICU, invasive ventilation, and death, was higher in those who had known comorbidities (4). In addition, one meta-analysis previously reported that certain comorbidities were more prevalent in severe COVID-19 patients (5).
This study is among the first investigations aiming to frame the current picture of the battle with the initial phase of the COVID-19 outbreak in one of Iran’s provinces. The main strength of this study is its timing, i.e., reporting the clinical patterns of hospitalized patients during the early period of the outbreak, when the medical system is presumed not to be overloaded. Additionally, acquiring data from the electronic data entry platform, which was designated exclusively to collect data of COVID-19 patients during their course of admission, along with the large sample size add to the robustness of the study results.
However, the findings on the clinical patterns and outcomes of the patients need to be interpreted in the context of our limitations. Among these caveats, we must emphasize the low availability of PCR testing in Iran. In addition, we did not have access to data on the patients’ laboratory results, their smoking status, or the medications received. The external validity of our results needs further consideration. Our cohort study was conducted in all public and private sector hospitals in Qazvin province during the first three weeks since the epidemic began. Thereby, the generalization of our results to other populations should be carried out with caution.
The applied diagnosis and treatment flowchart, as was first proposed by the INIGCDT (3), merits further explanation. These guidelines, which have been mainly adapted from the World Health Organization (WHO) guidelines (6, 7), proposed chest imaging as the first diagnostic step to screen patients who require prompt hospitalization amid shortages in RT-PCR test kits in Iran. As a result, all admitted patients with suggestive COVID-19 symptoms, with or without comorbidities, underwent chest imaging (specifically a chest CT scan) in Qazvin hospitals. Among them, 59.3% were tested with RT-PCR, of whom 24.9% had negative results. This proportion of negative test results is in line with previous findings. While there has been a significant correlation observed between throat swab and sputum sample viral loads (8), one study examining the bio-distribution of SARS-CoV-2 in different body tissues reported positive RT-PCR rates in only 72% of sputum specimens (9).
Examining the concordance between chest CT scan and PCR test results, a previous study from Wuhan, China reported that chest CT sensitivity was 97% in RT-PCR-positive patients. On the other hand, in the PCR-negative patients, 75% had positive CT scan findings, 81% of whom were later considered as highly likely or probable cases of COVID-19 (10). Another study showed that the sensitivity of chest CT in diagnosing COVID-19 was significantly higher than RT-PCR in their patients (98 vs. 71%) (11).
When categorizing CT-diagnosed COVID-19 patients based on their RT-PCR test results, as expected, the worst scenario was reported in the group of patients with suggestive respiratory symptoms or underlying diseases who had positive PCR test results. These individuals accounted for 41% of ICU admissions throughout Qazvin province, with a 77% CFR, even after receiving ICU services. On the other hand, the group with negative PCR test results who had suggestive clinical symptoms or underlying diseases accounted for 32% of COVID-19-related ICU admissions in the province, among whom 71% recovered. It has been previously reported that negative PCR results may be due to lower viral load in patients’ specimens (12, 13), which could provide a possible explanation for the lower CFR among the test-negative group. In the “no-test” group, which comprised CT-positive patients who were not tested for COVID-19 despite having suggestive clinical symptoms or coexisting diseases, 3.6% were admitted to ICUs, of whom 66.7% eventually recovered. The overall CFR between no-test group and patients with negative PCR results did not differ significantly. Based on the results of multilevel logistic regression analysis, in the first weeks of the epidemic, although PCR testing was more reserved for patients in a critical condition, its results should be presumed that a rather preemptive transfer of PCR positive patients to the ICU would partially explain the lower CFR in this group. Therefore, the criteria for ICU admission and prioritized allocation of the limited ICU beds should be identified (Table 5).
| Group | In-Hospital Mortality (95% CI) | Odds Ratio (95% CI) | P-Value |
|---|---|---|---|
| Test negative/no ICU | 6.1 (5.7, 6.9) | Reference | |
| Test positive/no ICU | 19.0 (18.2, 19.8) | 4.0 (1.8, 8.9) | 0.001 |
| No test/no ICU | 3.5 (3.2, 3.7) | 0.5 (0.2, 1.3) | 0.202 |
| Test negative/ICU | 12.6 (11.9, 13.2) | 2.3 (0.5, 10.4) | 0.264 |
| Test positive/ICU | 44.4 (43.2, 45.6) | 16.8 (5.6, 49.7) | < 0.001 |
| No test/ICU | 12.2 (11.6, 12.8) | 2.2 (0.2, 20.7) | 0.467 |
Multi-level Regression Analysis to Compare in-Hospital Mortality Rate in Six Groups Based on PCR Test Results and ICU Admission
Additionally, there was a group of patients with negative PCR results who also lacked major clinical symptoms or significant comorbidities. These patients had the lowest CFR, and none of them were ICU-admitted. These non-COVID-19 hospitalized patients, who were not included in our analysis, may serve as a basis for comparing the admission course and outcomes of the study patients.
The importance of available ICU facilities in such an epidemic cannot be overemphasized. As officially announced by the Iranian Ministry of Health and Medical Education (MOHME) in 2018, there are a total of 8,264 ICU beds nationwide, and these were occupied by 453,891 patients during the year 2018 (occupancy rate: 55 patients/bed/year). In Qazvin province, which includes 1.6% of Iran’s population, there are 96 ICU beds available (7.7 beds/100,000 of the population), comprising 1.1% of the total ICU beds in Iran. In this study, among the 92 non-survivors treated outside ICUs, 63% presented with shortness of breath, and 66% had low pulse oximetry readings. Among the 612 patients who presented an SpO2 of < 93%, only 6.2% were admitted to ICUs. Considering that the ICU admission rate in this study was 5.2% (equal to 52 beds of total ICU beds in Qazvin province), it can be concluded that there is a need for at least 90 additional ICU beds throughout the province to save more lives. Interestingly, other studies have previously reported that while most COVID-19 patients present with a mild illness, about 14% progress to more severe forms of the disease and require hospitalization, with 5% needing ICU care (14-16).
We also need to briefly mention the RT-PCR testing process. Due to the considerable shortage of PCR test kits in Iran, and according to the INIGCDT, physicians were encouraged to perform the test only in patients who were in a critical condition. Moreover, no re-testing was provided for patients with negative tests. While it has been reported that COVID-19 patients with more severe forms of the disease have higher viral loads and longer periods of viral shedding (6), the aforementioned testing protocol may have affected the results of the association between positive PCR test results and CFR.
In conclusion, we observed that COVID-19 patients hospitalized with mild symptoms, despite having positive chest CT changes and major comorbidities, were more likely to have negative rRT-PCR test results. Hence, there was a lower CFR and a more favorable outcome. Conversely, positive rRT-PCR test results were more prevalent in patients presenting with low SpO2 or unconsciousness, and they were strongly associated with increased odds of death among chest CT-positive patients. Considering the serious shortage in ICU capacity, preemptive transfer of more vulnerable rRT-PCR test-positive patients to the ICU might save their lives.