1. Background
COVID-19 was first identified in China in December 2019 and quickly spread to many countries (1). From the first days of this epidemic, respiratory symptoms were reported as the main symptoms of the disease, but with the spread of COVID-19, new manifestations such as gastrointestinal involvement (2), cardiac involvement (3), nervous system complications, and loss of smell and taste (4) were gradually observed in some patients and reported in studies. Preliminary studies also found probable evidence suggesting the presence of some electrolyte imbalances in these patients (5, 6), but no consistent and specific report on electrolyte imbalances in these patients is available.
In managing hospitalized COVID-19 patients, we noticed numerous cases of electrolyte imbalances, hypocalcemia in particular, especially among the severe cases of the disease. Given the importance of monitoring and correcting electrolyte imbalances in providing patients better care, attention to electrolyte imbalances is always crucial. Moreover, the side effects of the medications used in the putative treatment of this disease, including cardiac complications (long QT due to hydroxychloroquine), may be exacerbated by electrolyte imbalances (7).
Various mechanisms, including the effect of inflammatory proteins, the role of vitamin D, the effects of angiotensin, or electrolyte loss through the digestive may play a role in the electrolyte changes in the course of COVID-19, and further studies on this topic can contribute to a better understanding of the pathophysiology of the disease. Moreover, given the exacerbation of ARDS in the presence of some electrolyte imbalances, including hypokalemia, a relationship may exist between the severity and mortality of COVID-19 and these imbalances.
2. Objectives
Given the limited number of studies conducted on the prevalence of electrolyte imbalances among patients with COVID-19, the present study aimed to examine the prevalence of electrolyte imbalances in these patients and investigate the relationship between possible electrolyte imbalances and disease severity at the time of admission for better care of the patients during this crisis. Furthermore, the identification of electrolyte imbalances in this disease may, to some extent, help elucidate the unknown mechanisms and pathophysiology of this novel disease.
3. Methods
In this retrospective cross-sectional study, the study population consisted of adult patients (≥ 16 years) with a definitive diagnosis of COVID-19 by RNA detection of the SARS-COV-2 or lung involvement on chest CT scan, admitted to Imam Khomeini Hospital from March 20th to May 20th, 2020. In cases where polymerase chain reaction (PCR) was not available or was negative, lung involvement in favor of COVID-19 was considered the basis of diagnosis. Pediatrics less than 16 years or patients with a history of severe lung disease, end stage renal disease, hemodialysis, previous electrolyte disturbances, or hereditary hypophosphatemia were excluded from the study.
Upon admission of the patients, in the first visit, a blood sample was sent for the analysis of the electrolytes (calcium, potassium, sodium, magnesium, and phosphorus). The results of the analysis were used in the study of the prevalence of electrolyte imbalances (hypo- and hypercalcemia, hypo- and hyperkalemia, hypo- and hypernatremia, hypo- and hypermagnesemia, and imbalances in the phosphorus level). The normal level of sodium, potassium, calcium, magnesium, and phosphorus was 135 - 145 mEq/L, 3.5 to 5 mEq/L, 8.5 - 10.5 mg/dL, 1.3 to 2.1 mEq/L, and 2.5 to 4.5 mg/dL respectively, and values below and upper this ranges were considered as hypo- and hyper-imbalances. In addition, the albumin levels were measured simultaneously to determine the corrected calcium levels, and the patient's serum calcium levels were corrected using the serum albumin level. Each one g/dL reduction in the serum albumin concentration will lower the total calcium concentration by approximately 0.8 mg/dL. The normal albumin parameter is set to 4 g/dL. All data in the questionnaires were analyzed in SPSS 25, and the prevalence of electrolyte imbalances among the patients was determined.
Based on the WHO guideline (8), the criteria of respiratory rate and arterial blood oxygen saturation at the time of the visit were used to assess disease severity. COVID-19 patients with fever or suspected respiratory infection, respiratory rates of > 30 per minute, arterial blood oxygen saturation levels of ≤ 93% at room air, or severe respiratory distress were regarded as severe cases. Finally, the relationship between electrolyte imbalances and disease severity, ICU admission, and mortality was evaluated using an independent t-test. Moreover, considering the possible effects of underlying diseases or the use of various medications on the occurrence of electrolyte imbalances, the relationship between these two factors and electrolyte imbalances was examined.
4. Results
Of 1072 hospitalized patients studied, 657 (61.3%) were men, and 415 (38.7%) were women. The mean age of the patients was 57.8 years, with a minimum of 16 and a maximum of 101 years (Table 1).
| Variables | No. (%) |
|---|---|
| Total | 1072 (100) |
| Gender | |
| Male | 657 (61.3) |
| Female | 415 (38.7) |
| Age (y) | |
| < 20 | 1 (0.1) |
| 20 - 39 | 158 (14.7) |
| 40 - 59 | 391 (36.5) |
| 60 - 79 | 408 (38.7) |
| > 80 | 99 (9.2) |
| Missing data | 15 (1.4) |
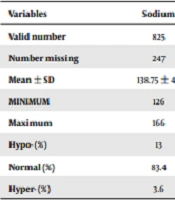
In hospitalized COVID-19 patients, the prevalence of hypocalcemia was higher than the other electrolyte imbalances. The mean serum sodium level was 138.7 ± 4.1 mEq/L. Hyponatremia was observed in 13% and hypernatremia in only 3.6% of the cases. In the other cases, sodium levels were in the normal range. The mean potassium level was 4.2 ± 0.6 mEq/L, and 6.4% and 8.5% of the patients were hypokalemic and hyperkalemic, respectively. The mean albumin-corrected serum calcium level was 8.56 ± 0.75 mg/dL, and 47.7% of the patients had hypocalcemia, while only 1.1% had hypercalcemia. Calcium was normal in the rest (51.1%) of the cases. Only 272 patients had serum albumin in their medical records, and the mean serum albumin level was 3.62 ± 0.59 g/dL. The mean serum magnesium and phosphorus levels were 2.02 ± 0.3 mg/dL and 3.2 ± 0.9 mg/dL, respectively. Moreover, 15.8% and 21.1% of the patients had hypomagnesemia and hypophosphatemia, respectively. In all electrolyte disorders except potassium, the prevalence of decreased serum levels (Hypo-) was greater than their increase (Hyper-) (Table 2).
| Variables | Sodium | Potassium | Calcium | Corrected Calcium | Magnesium | Phosphorus |
|---|---|---|---|---|---|---|
| Valid number | 825 | 861 | 582 | 264 | 651 | 525 |
| Number missing | 247 | 211 | 490 | 808 | 421 | 547 |
| Mean ± SD | 138.75 ± 4.1 | 4.25 ± 0.6 | 8.38 ± 0.7 | 8.56 ± 0.75 | 2.02 ± 0.33 | 3.2 ± 0.97 |
| MINIMUM | 126 | 2.3 | 5.8 | 6.76 | 0.4 | 0.8 |
| Maximum | 166 | 7.7 | 10.7 | 11.6 | 3.4 | 8.0 |
| Hypo- (%) | 13 | 6.4 | 54.6 | 47.7 | 15.8 | 21.1 |
| Normal (%) | 83.4 | 85.1 | 44.5 | 51.1 | 80.5 | 70.5 |
| Hyper- (%) | 3.6 | 8.5 | 0.9 | 1.1 | 3.7 | 8.4 |
In total, 12.4% of the patients were hospitalized in the ICU, and the rest were in the ward. Regarding disease severity, as measured by the respiratory rate and arterial blood oxygen saturation in room air, 77.3% of the COVID-19 patients had severe and 22.7% mild disease. A respiratory rate of > 30 per minute was observed in 8.6% of the hospitalized patients, and arterial blood oxygen saturation of ≤ 93% was found in 73% of them. Mean serum sodium, calcium, and magnesium levels were lower in severe cases, ICU hospitalization, and deceased than in non-severe cases. However, these differences were only significant in terms of sodium levels. As shown in Table 3, in all severe or non-severe cases, calcium levels were below normal or in the lower limit of the normal range. Higher levels of potassium and phosphorus were seen in more severe cases, ICU hospitalization, and deceased (Table 3).
| Variables | Sodium | P Value | Potassium | P Value | Calcium | P Value | Corrected Calcium | P Value | Magnesium | P value | Phosphorus | P Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severity | 0.036 b | 0.441 | 0.646 | 0.489 | 0.145 | 0.504 | ||||||
| Severe | 138.47 ± 4.19 | 4.27 ± 0.61 | 8.37 ± 0.68 | 8.58 ± 0.67 | 1.98 ± 0.28 | 3.24 ± 1.03 | ||||||
| Non- severe | 139.93 ± 3.70 | 4.23 ± 0.57 | 8.46 ± 0.71 | 8.60 ± 0.77 | 2.02 ± 0.33 | 3.17 ± 0.91 | ||||||
| ICU admission | 0.028 b | 0.002 b | 0.736 | 0.923 | 0.465 | 0.467 | ||||||
| ICU | 138.28 ± 5.19 | 4.38 ± 0.81 | 8.02 ± 0.65 | 8.54 ± 0.74 | 1.97 ± 0.31 | 3.28 ± 0.96 | ||||||
| Ward | 138.94 ± 3.90 | 4.21 ± 0.55 | 8.42 ± 0.65 | 8.57 ± 0.79 | 2.13 ± 0.32 | 3.19 ± 1.04 | ||||||
| Mortality | 0.001 b | 0.003 b | 0.289 | 0.91 | 0.03 b | 0.001 b | ||||||
| Deceased | 138.62 ± 6.15 | 4.57 ± 0.87 | 8.09 ± 0.78 | 8.48 ± 0.71 | 1.99 ± 0.32 | 3.67 ± 1.29 | ||||||
| Alive | 138.94 ± 3.73 | 4.19 ± 0.53 | 8.43 ± 0.67 | 8.86 ± 0.93 | 2.13 ± 0.35 | 3.15 ± 0.91 |
a Values are expressed as mean ± SD.
b P value < 0.05.
The relationship between the electrolyte imbalances and underlying diseases (hypertension, diabetes, cardiovascular disease, chronic kidney disease (CKD), cirrhosis, pulmonary diseases, and underlying malignancies) was investigated, as there was the possibility of the electrolyte imbalance being due to underlying diseases. Hyperkalemia was significantly higher in patients with cardiovascular disease (P = 0.018). Diabetes patients were significantly more likely to have hypernatremia (P = 0.012). Hyperphosphatemia was significantly higher in patients with CKD and those with cardiovascular disease (P = 0.016 and P = 0.001, respectively). The relationship between electrolyte imbalances and blood pressure-regulating drugs and steroids was also examined. The only significant relationship found was between the use of blood pressure-regulating drugs and hyperkalemia (P = 0.034).
5. Discussion
As noted before, various aspects of the manifestations of COVID-19, such as pulmonary, inflammatory, digestive, and nervous manifestations, have been addressed in previous studies. However, few studies have specifically examined electrolyte imbalances seen in the course of this disease. The following four factors further demonstrate the importance of discussing electrolyte imbalances in COVID-19: (1) closer attention to electrolyte imbalances in the course of this disease and their timely correction can contribute to the better management of these patients; (2) there still is no specific treatment available for this disease, and in some centers, drugs such as hydroxychloroquine, lopinavir/ritonavir (Kaletra), and steroids are used in the treatment, which can have side effects similar to those of electrolyte imbalances (such as long QT in the case of hypocalcemia and hydroxychloroquine), or cause electrolyte imbalances (in the case of steroids); (3) identification of the more prevalent electrolyte imbalances in this disease and confirmation of the findings in multiple studies may help elucidate the pathophysiology of COVID-19; (4) electrolyte imbalances can be associated with disease severity and help establish disease prognosis.
As a result of this study, hypocalcemia was more prevalent than other electrolyte imbalances among hospitalized COVID-19 patients with a prevalence of 47.7%, followed by hypophosphatemia (21.1%), hypomagnesemia (15.8%), and hyponatremia (13%), all of which had a prevalence greater than 10%. In a review study by Lippi et al. on electrolyte imbalances in COVID-19 in 1415 patients, hypocalcemia, hyponatremia, and hypokalemia were more prevalent than other electrolyte imbalances. There was no mention of magnesium or phosphorus levels in their study, which examined only sodium, potassium, calcium, and chlorine levels. Moreover, in the five studies reviewed by Lippi et al., calcium levels were reported as total calcium uncorrected by albumin levels, a point which may cause problems in the interpretation of the test results (9); however, in the present study, efforts were made to resolve this issue by correcting calcium levels in accordance with serum albumin levels.
Hypocalcemia in COVID-19 patients can be attributed to various mechanisms, including a reduction in albumin in inflammation, mechanisms resulting in hypocalcemia in critically ill patients, or the potential role of vitamin D deficiency in disease pathogenesis. Given the role of albumin as a negative acute phase protein, the possible decrease in blood calcium levels can be attributed to the decrease in total calcium levels due to the decline in albumin levels during the acute inflammation caused by COVID-19. Acute phase proteins are a group of plasma proteins whose level in blood is increased or decreased by at least 25% at the time of inflammation. Ferritin, plasmin, von Willebrand factor, and hepcidin are among the proteins that increase (positive) during inflammation (10), while transferrin and albumin are among the acute phase proteins decreasing during inflammation (negative) (11); therefore, a reduction in serum albumin can be one of the possible causes of the decrease in the level of total serum calcium.
In this study, after correcting calcium levels according to albumin serum levels, still, about 50% of the patients had corrected hypocalcemia; thus, it seems that mechanisms other than albumin decrease must have played a role in the situation. One of the mechanisms proposed as the cause of hypocalcemia in critically ill patients, those with sepsis, and those with severe burns is the disturbance in PTH secretion, a decrease in calcitriol production, and PTH resistance (12, 13). This mechanism may play a role in hospitalized COVID-19 patients, too. The possible basic mechanism could be related to hypomagnesemia and the inflammatory effects of cytokines on the parathyroid gland, kidneys, and bones. Some studies have reported about 80-90% prevalence of hypocalcemia in critically ill patients or post-surgery (14). Furthermore, given the effects of vitamin D deficiency mentioned in the COVID-19 crisis, this factor may also be implicated in the high prevalence of hypocalcemia. Various studies have shown that vitamin D can reduce the risk of respiratory infections such as influenza and COVID-19 by regulating inflammatory cytokines and increasing cathelicidins and defensins (15, 16). Past studies have also noted vitamin D deficiency as a risk factor for ARDS and ARDS severity (17).
The relatively high prevalence of hypophosphatemia and hypomagnesemia may also be related to vitamin D deficiency, and more studies are needed in this regard. Given the relatively high prevalence of nausea and diarrhea, the digestive loss of electrolytes can also cause electrolyte decrease in the serum (18). In the 2020 demographic study by Qian et al. on 91 hospitalized patients in China, hyponatremia (17.58%) and hypocalcemia (12.09%) were reported more frequently than other imbalances. Calcium was higher than the normal range in only one of the 91 patients. Hypokalemia was observed in 7.96% of the patients, but potassium was not over 5.3 mEq/L in any of the patients. Furthermore, in that study, hypocalcemia was shown to have a relationship with the more severe disease cases (P = 0.0056) (19).
Our study showed that mean serum sodium, calcium, and magnesium levels were lower in severe cases, ICU hospitalization, and deceased than in non-severe cases. About calcium, as shown in Table 3, in all severe or non-severe cases, calcium levels were below normal or in the lower limit of the normal range. Higher levels of potassium and phosphorus were seen in more severe cases, ICU hospitalization, and death. However, in the review study by Lippi et al., disease severity was significantly associated with hyponatremia, hypocalcemia, and hypokalemia (9). They mentioned the effects of hypokalemia on exacerbating ARDS to explain the relationship between hypokalemia and disease severity. It has also been mentioned that the higher prevalence of hypokalemia in these patients could be due to the SARS-CoV-2 virus binding to the angiotensin-converting enzyme 2 (ACE2) receptor and reducing the ACE2 expression that, eventually, leads to an increase in angiotensin II level, which, in turn, increases renal potassium excretion and results in hypokalemia (12). In the present study, the difference between the prevalence of hypokalemia (6.4%) and hyperkalemia (8.5%) was small. However, in our study, contrary to the study by Lippi et al. (9), hyperkalemia had a higher prevalence among the patients, especially in severe cases. Given the significant relationship between hyperkalemia and cardiovascular diseases in our study (P = 0.018), underlying conditions could be the cause of the relationship between hyperkalemia and disease severity, ICU admission, and increased mortality in the patients, and more studies need to be conducted on the subject, with removing the effect of comorbidities.
In this study, disease severity was defined according to the WHO guidelines, i.e., a respiratory rate > 30 per minute or arterial blood oxygen saturation ≤ 93% demonstrated the severity of COVID-19 pneumonia in patients with pulmonary involvement. Accordingly, among the studied hospitalized patients, 78.1% had severe, and 21.9% had mild diseases. In the review study by Lippi et al. (9), only 244 out of 1415 patients had severe disease. This difference is due to, firstly, the difference in study populations in the two studies (out-patients and hospitalized patients in the study by Lippi et al. (9) and only hospitalized patients in our study), and secondly, the disease severity criteria selected in the two studies.
Finally, it is recommended that more attention is paid to the monitoring and correcting electrolyte imbalances in hospitalized COVID-19 patients because of the high prevalence of these imbalances, especially hypocalcemia, and the risk of their interaction with some of the drugs used in treating the disease. It is also suggested that more studies should be conducted on this topic by removing the effects of underlying factors, such as underlying diseases and the use of drugs, to obtain a more accurate picture of the prevalence of electrolyte imbalances in COVID-19 so that better care for the patients can be provided and the pathophysiology of this novel disease can be better understood.
This study has some limitations. First, owing to the retrospective nature of these data, all patients' data were incomplete in terms of electrolytes and albumin. Therefore, we did not have information about all the patients regarding calcium, magnesium, and corrected calcium. Second, we excluded patients with ESRD or a history of severe electrolyte disturbances, but our data were based on medical records, and the possibility of error is inevitable. We did not have enough information about the recent serum, insulin, or corticosteroid therapy that could affect electrolytes, and we did not exclude these groups of patients.
5.1. Conclusions
Because of the high prevalence of electrolyte imbalance in hospitalized COVID-19 patients and the interactions of electrolyte imbalance with some drugs used in its treatment, it is recommended that electrolytes be regularly monitored and corrected in COVID-19 patients to ensure better care for them. Moreover, since the number of studies conducted on electrolyte imbalances in COVID-19 is limited, it is suggested that more studies be conducted on this topic.