1. Background
Streptococcus pneumoniae is one of the main causes of infections, such as acute otitis media, sepsis, bacteremia, meningitis, and pneumonia. Invasive pneumococcal disease (IPD) is a severe pneumococcal infection that is responsible for a high rate of morbidity and mortality, especially in immunocompromised individuals, young children, and seniors (1). Pneumonia annually kills 0.7 to 1 million children under 5 years of age, according to the World Health Organization (WHO). It can be said that this infection is one of the main causes of child mortality in developing countries and adult mortality worldwide (2).
Polysaccharide capsules are the most important antigens of S. pneumoniae. Pneumococcal vaccines are produced based on these capsules. The 7-valent pneumococcal conjugate vaccine (PCV-7) was the first vaccine to be approved by the U.S. Food and Drug Administration in 2000. After the introduction of PCV-7, this vaccine was replaced by two higher-valent vaccines, 10-valent pneumococcal conjugate vaccine (PCV-10) and 13-valent pneumococcal conjugate vaccine (PCV-13), to cover a wider range of serotypes. The PCV-13 contains strains 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F. Additionally, Pneumococcal PolySaccharide Vaccine23 (PPSV23) includes strains 1, 2, 3, 4, 5, 6B, 7F, 8, 9V, 9N, 10A, 11A, 12F, 14A, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F. The immunogenicity of the polysaccharide vaccine is lower than that of the conjugate vaccine (3).
The National Immunization Survey data indicate that in 2009, PCV-7 coverage in children aged 19 - 35 months was 92.6% after receiving ≥ 3 doses and 80.4% after receiving ≥ 4 doses (4). Currently, the WHO recommends the universal program of PVC administration to children. Therefore, the data on dispensing pneumococcal serotypes is of great importance for the assessment of the coverage of the current vaccines (5).
The pneumococcal disease incidence and mortality rates have been reduced by about 5 - 40% of all-cause pneumonia among different children age groups after pneumococcal conjugate vaccine introduction. Non-IPDs are sinusitis and acute otitis media. Noninvasive infections are caused by the isolation of S. pneumoniae from nonsterile sites, such as sputum, urine, skin, and eyes. On the other hand, IPD causes infections in sterile sites of the body, including the bloodstream, cerebrospinal fluid, and pleural fluid. The coverage of invasive serotypes, nasopharyngeal colonization, and antibiotic resistance can be related to age, geographical location, and economic conditions of the population (6). Specific pathogen serotyping provides significant epidemiological information for the production of proper vaccine compounds.
2. Objectives
This study aimed to determine S. pneumoniae serotypes isolated from the clinical samples of Iran. It might be a preliminary study for the production of a native vaccine in Iran in the future.
3. Methods
3.1. Study Design
In this study, all samples were imported from patients of any age with IPD infection. Pneumococcal isolates were collected from the typical sterile clinical specimens from the hospitals in five provinces of Northern, Southern, Eastern, Western, and Central Iran. The selected hospitals had microbiology laboratories and were distributed almost equally throughout Iran. The identified strains through the transport environment were sent to the Immunology Research Center of Iran University of Medical Sciences, Tehran, Iran, by mentioning the patients’ demographic characteristics, including age, gender, and isolated location.
3.2. Culture
The S. pneumoniae strains were subcultured on enriched chocolate agar plates. The plates were incubated overnight at 37 °C under 5% CO2 (4).
3.3. DNA Extraction
A culture test and polymerase chain reaction (PCR) were carried out to evaluate the presence of S. pneumoniae in the samples. At first, genomic deoxyribonucleic acid (DNA) was extracted from the specimens using Sinaclon Kit (Sinaclon, Iran) according to the manufacturer’s instructions. The optical density of the extracted DNA was detected at 260 nanometers using a NanoDrop1000 spectrophotometer (Thermo Fisher Scientific, USA).
3.4. Multiplex PCR
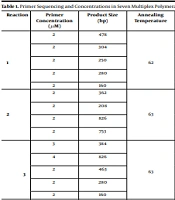
The primers used in this study were classified into seven multiplex reactions (Table 1). For the identification of pneumococcal serotypes, each reaction of multiplex PCR assay contained four sets of serotype-specific primers for four various serotypes and one internal positive control that targeted all known pneumococcal capsular polysaccharide operons. The PCR assays were performed in 25 μL volumes, with each reaction mixture containing PCR Master Mix (Sinaclon, Iran) and primers with concentrations as specified in Table 1. The crude extract (2.5 μL) was used as the DNA template for each PCR. The PCR was performed in a thermal cycler (SenoQuest, Germany) under the conditions, including 94°C for 4 minutes followed by 30 amplification cycles at 94°C for 46 seconds, 54°C for 45 seconds, and 65°C for 2 minutes. Gels were stained with a safe DNA stain (Sinaclon, Iran). The template of the PCR products was distinguished by comparing to the molecular size standard (50-bp ladder, Sinaclon, Iran) (4, 7).
| Reaction | Primer Concentration (μM) | Product Size (bp) | Annealing Temperature | Primers | Sequence Primer 5' to 3' | |
|---|---|---|---|---|---|---|
| 1 | 2 | 478 | 62 | 19A | F -GTT AGT CCT GTT TTA GAT TTA TTT GGT GAT GT | R- GAG CAG TCA ATA AGA TGA GAC GAT AGT TAG |
| 2 | 304 | 19F | F -GTT AAG ATT GCT GAT CGA TTA ATT GAT ATC C | R-GTA ATA TGT CTT TAG GGC GTT TAT GGC GAT AG | ||
| 2 | 250 | A/B | F- AAT TTG TAT TTT ATT CAT GCC TAT ATC TGG | R-TTA GCG GAG ATA ATT TAA AAT GAT GAC TA | ||
| 2 | 280 | 6 | F -CTC TAT AGA ATG GAG TAT ATA AAC TAT GGT TA | R-CCA AAG AAA ATA CTA ACA TTA TCA CAA TAT TGG C | ||
| 2 | 160 | 1 | ||||
| 2 | 2 | 362 | 63 | 5 | F-ACTT GGC GCA GGT GTC AGA ATT CCC TCT AC | R-GCC AAA ATA CTG ACA AAG CTA GAA TAT AGC C |
| 2 | 208 | 14 | F-RTA CCT ACA CAA CTT CTG ATT ATG CCT TTG TG | R-GCT CGA TAA ACA TAA TCA ATA TTT GAA AAA GTA TG | ||
| 2 | 826 | 7F | F-CCT ACG GGA GGA TAT AAA ATT ATT TTT GAG | R-CAA ATA CAC CAC TAT AGG CTG TTG AGA CTA AC | ||
| 2 | 753 | 9V | F-CTT CGT TAG TTA AAA TTC TAA ATT TTT CTA AG | R-GTC CCA ATA CCA GTC CTT GCA ACA CAA G | ||
| 3 | 3 | 384 | 63 | F23 | F- GTA ACA GTT GCT GTA GAG GGA ATT GGC TTT TC | R-CAC AAC ACC TAA CAC ACG ATG GCT ATA TGA TTC |
| 4 | 826 | 7F | F- CCT ACG GGA GGA TAT AAA ATT ATT TTT GAG | R-CAA ATA CAC CAC TAT AGG CTG TTG AGA CTA AC | ||
| 2 | 463 | 11A | F-GGA CAT GTT CAG GTG ATT TCC CAA TAT AGT G | R-GAT TAT GAG TGT AAT TTA TTC CAA CTT CTC CC | ||
| 2 | 280 | 1 | F-CTC TAT AGA ATG GAG TAT ATA AAC TAT GGT TA | R-CCA AAG AAA ATA CTA ACA TTA TCA CAA TAT TGG C | ||
| 2 | 160 | cps | ||||
| 4 | 4 | 988 | 62 | 16F | F-TTG GAA TTT TTT AAT TAG TGG CTT ACC TA | R-CAT CCG CTT ATT AAT TGA AGT AAT CTG AAC C |
| 2.5 | 573 | 18C | F-CTT AAT AGC TCT CAT TAT TCT TTT TTT AAG CC | R-TTA TCT GTA AAC CAT ATC AGC ATC TGA AAC | ||
| 2 | 677 | 35B | F-GAT AAG TCT GTT GTG GAG ACT TAA AAA GAA TG | R-CTT TCC AGA TAA TTA CAG GTA TTC CTG AAG CAA G | ||
| 2 | 376 | 12F | F-GCA ACA AAC GGC GTG AAA GTA GTT G | R-CAA GAT GAA TAT CAC TAC CAA TAA CAA AAC | ||
| 5 | 3 | 294 | 61 | 8 | F-GAT GCC ATG AAT CAA GCA GTG GCT ATA AAT C | R-ATC CTC GTG TAT AAT TTC AGG TAT GCC ACC |
| 3 | 371 | 3 | F-ATG GTG TGA TTT CTC CTA GAT TGG AAA GTA G | R-CTT CTC CAA TTG CTT ACC AAG TGC AAT AAC G | ||
| 3 | 496 | 15B5B | F-ATT AGT ACA GCT GCT GGA ATA TCT CTT C | R-GAT CTA GTG AAC GTA CTA TTC CAA AC | ||
| 4 | 701 | 31 | F-GGA AGT TTT CAA GGA TAT GAT AGT GGT GGTGC | R-CCG AAT AAT ATA TTC AAT ATA TTC CTA CTC | ||
| 6 | 3 | 280 | 60 | 1 | F-CTC TAT AGA ATG GAG TAT ATA AAC TAT GGT TA | R-CCA AAG AAA ATA CTA ACA TTA TCA CAA TAT TGG C |
| 3 | 628 | 10A | F-GGT GTA GAT TTA CCA TTA GTG TCG GCA GAC | R-GAA TTT CTT CTT TAA GAT TCG GAT ATT TCT C | ||
| 3 | 517 | 35F | F-GAA CAT AGT CGC TAT TGT ATT TTA TTT AAA GCA A | R-GAC TAG GAG CAT TAT TCC TAG AGC GAG TAA ACC | ||
| 4 | 408 | 34 | F-GCT TTT GTA AGA GGA GAT TAT TTT CAC CCA AC | R-CAA TCC GAC TAA GTC TTC AGT AAA AAA CTT TAC | ||
| 7 | 2 | 514 | 63 | 20 | F-GAG CAA GAG TTT TTC ACC TGA CAG CGA GAA G | R-CTA AAT TCC TGT AAT TTA GCT AAA ACT CTT ATC |
| 2 | 260 | 7C | F-CTA TCT CAG TCA TCT ATT GTT AAA GTT TAC GAC GGG A | R-GAA CAT AGA TGT TGA GAC ATC TTT TGT AAT TTC | ||
| 2 | 693 | 17F | F-TTC GTG ATG ATA ATT CCA ATG ATC AAA CAA GAG | R-GAT GTA ACA AAT TTG TAG CGA CTA AGG TCT GC | ||
| 2 | 430 | 4 | F- CTG TTA CTT GTT CTG GAC TCT CGA TAA TTG G | R-GCC CAC TCC TGT TAA AAT CCT ACC CGC ATT G | ||
Abbreviation: cps, capsular polysaccharide.
3.5. Statistical Analysis
The chi-square test was used to compare the proportions. Statistical analyses were performed using SPSS software (version 22.0). P-values less than 0.05 were considered statistically significant.
4. Results
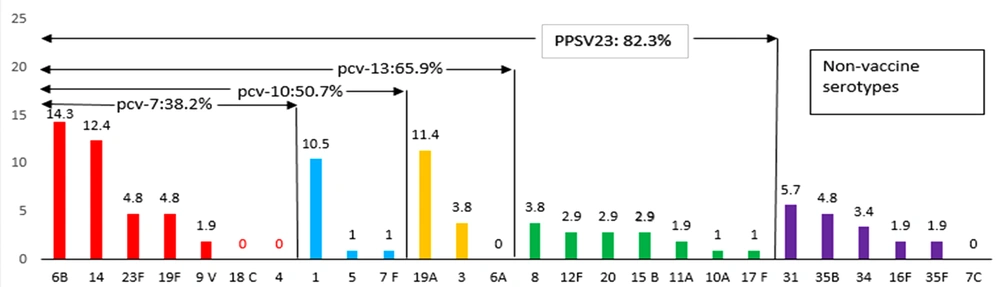
Out of 105 isolated pneumococcal strains, 13%, 36%, 1.5%, 13%, 3%, 1.5%, 12%, 15%, and 5% were from the blood, cerebrospinal fluid, urine, trachea, abscess, throat, bronchoalveolar lavage, eye, and pleural fluid, respectively. The 105 strains were serotyped into 22 serogroups using the PCR multiplex technique. Overall, 22 serotypes were identified among the whole isolates. The identified isolates included serotypes 6B (14.3%), 14 (12.4%), 19A (11.3%), 1 (10.5%), 31 (5.7%), 23F (4%), 19F (4%), 35B (4.8%), 3 (3.8%), 8 (3.8%), 34 (3.4%), 12F (2.9%), 20 (2.9%), 15B (2.9%), 9V (1.9%), 11A (1.9%), 16F (1.9%), 35F (1.9%), 5 (1%), 7F (1%), 10A (1%), and 17F (1%). However, serotypes 18C, 4, and 7C were not observed (Figure 1). Four serotypes were most prevalent among the clinical isolates, namely 6B (14.3%), 14 (12.4%), 19A (11.3%), and 1 (10.5%).
Distribution pattern of serotypes of Streptococcus pneumoniae and vaccine coverage rates for 7-valent pneumococcal conjugate vaccine (PCV-7), 10-valent pneumococcal conjugate vaccine (PCV-10), 13-valent pneumococcal conjugate vaccine (PCV-13), and pneumococcal polysaccharide vaccine (PPSV23); each bar illustrating the relative frequency of each serotype of S. pneumoniae; Value in the arrows above the bars illustrating vaccine coverage rates for nonvaccine serotypes of PCV-7, PCV-10, PCV-13, and PPSV23, respectively.
The isolated serotypes consisted of vaccine (83.5%) and nonvaccine (16.5%) serotypes. Table 2 shows the prevalence of serotypes in the studied strains between IPD and non-IPD. As shown in Table 2, 83% and 84% of serotypes isolated from IPD and non-IPD are vaccine serotypes. Nonvaccine serotypes included 16F (2.3%), 34 (2.3%), 31 (3.5%), 35B (5.9%), and 35F (2.3%). Twenty serotypes were identified among the whole isolates, including serotypes 6B (14.1%), 14 (12.9%), 1 (9.4%), 19A (8.2%), 15B (8.2%), 23F (5.9%), and 35B (5.9%) (Table 3). The most prevalent serotype among the clinical isolates (n = 85) was serotype 6B (14.1%). Additionally, serotype 6B was significantly more prevalent (P < 0.05) among the invasive (75%; 9/12) clinical isolates, compared to that among noninvasive (25%; 3/12) counterparts. Moreover, the serotype 19A was the most prevalent serotype in the noninvasive (16.1%; 5/31) clinical isolates (Table 3).
| Vaccine Type | IPD (n = 54) | Non-IPD (n = 31) | N = 85 (Total) |
|---|---|---|---|
| Vaccine serotype | 45 (83) | 26 (84) | 71 (83.5) |
| Nonvaccine serotype | 9 (17) | 5 (16) | 14 (16.5) |
Abbreviation: IPD, invasive pneumococcal disease.
a Values are expressed as No. (%).
| Variables | Serotype | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6B | 14 | 23F | 19F | 9V | 8 | 1 | 7f | 19A | 3 | 12F | 20 | 15B | 11A | 17F | 16F | 31 | 34 | 35B | 35F | Total | P-Value | |
| Invasive | 9 (16.6) | 7 (12.9) | 3 (5.5) | 3 (5.5) | 2 (3.7) | 1 (1.8) | 4 (7.4) | 2 (3.7) | 2 (3.7) | 1 (1.8) | 2 (3.7) | 0 | 6 (11.1) | 3 (5.5) | 0 | 2 (3.7) | 2 (3.7) | 3 (5.5) | 1 (1.8) | 1 (1.8) | 54 | <0.05 |
| Noninvasive | 3 (9.7) | 4 (12.9) | 2 (6.4) | 1 (3.2) | 0 | 0 | 4 (12.9) | 1 (3.2) | 5 (16.1) | 3 (9.6) | 0 | 1 (3.2) | 1 (3.2) | 0 | 1 (3.2) | 0 | 0 | 0 | 4 (12.9) | 1 (3.2) | 31 | |
| Total | 12 (14.1) | 11 (12.9) | 5 (5.9) | 4 (4.7) | 2 (2.3) | 1 (1.2) | 8 (9.4) | 3 (3.5) | 7 (8.2) | 4 (4.7) | 2 (2.3) | 1 (1.2) | 7 (8.2) | 3 (3.5) | 1 (1.2) | 2 (2.3) | 2 (2.3) | 3 (3.5) | 5 (5.9) | 2 (2.3) | 85 | |
a Values are expressed as No. (%).
5. Discussion
According to the results, all the bacteria identified based on phenotypic characteristics as S. pneumoniae were also confirmed by PCR. Moreover, the capsular polysaccharide A (CPSA) gene of S. pneumoniae was amplified by PCR. This finding is significant because it indicates that the CPSA gene is detectable in different pneumococcal strains. Therefore, it might be possible to use this method as a reliable and rapid method to identify pneumococcal strains in clinical specimens. The increase of PCR methods for the direct detection of elect serotypes from clinical specimens could be valuable in surveillance, especially when culture is not sufficiently sensitive (8).
The PCV-7 against the most common serotypes of IPD, including 4, 6B, 9V, 14, 18C, 19F, and 23F, has been available since 2001 and has led to a significant reduction in vaccine-type IPD in countries that started the childhood vaccination program (9, 10). Alizadeh Chamkhaleh et al. in a review study showed that among serotypes causing IPD, the serotype 23F (16.4%) was the most circulating serotype followed by 19F (15.2%), 19A (11.3%), 6A/B (9.2%), 9V (5.8%), and 11A (5.14%) (11). However, the most circulating serotypes observed in the present study were 6B (14.1%), 14 (12.9%), 1 (9.4%), 19A (8.2%), 15B (8.2%), 23F (5.9%), 35B (5.9%), 19F (4.7%), 9V (2.3%), and 11A (3.5%).
Serotypes 6B, 14, 19A, 15B, 1, 23F, 19F, 35B, 7F, and 34 were the most prevalent isolated serotypes. These 10 serotypes accounted for 75.1% of all the serotypes detected in the current study. However, in Hajia et al.’s study, serotypes 19 (8.2%), 6 (7.6%), 14 (7.2%), 19F (7.2%), 17 (6.4%), 21 (5.3%), 20 (5.1%), 12F (4.8%), 11 (4.5%), and 3 (4.3%) were the most common isolated serotypes, respectively, accounting for about 60% of the 14 studied serotypes (12). However, except for serotypes 19F and 14, the other serotypes are not similar to the predominant serotypes of the current study. Moreover, the prevalence of the serotype 19F in the present study was about twice as high as that in the Hajia et al.’s study (12). In comparison to Hajia et al.’s study, the prevalence of serotype 14 was doubled in the current study. This difference could be due to the study of populations from different regions of Iran. In Houri et al.’s study (13), the most commonly observed serotypes isolated from the nasopharyngeal samples of healthy children and determined by PCR were 19 (11.86%) and 6 (10%). Nonetheless, in the current study, the samples were isolated from patients with pneumococcal infections. However, according to the aforementioned Iranian studies and the present study, three serotypes of 19, 23, and 6 appear to be the most prevalent types in Iran.
The PCV-7 is reported to be highly effective worldwide; nevertheless, Iran, in comparison to the United States, has the lower serotype coverage for this vaccine (14), which highlights the importance of developing three new conjugate vaccines, namely PCV-10, PCV-13, and PPSV23, to include a larger number of serotypes. The PCV-13 contains serotypes 1, 3, 5, 6A, 7F, and 19A, from which serotypes 1, 3, and 5 are prevalent in Europe, Asia, and Africa. Most of these serotypes have emerged following the application of PCV-7 in these regions (15). Currently, no pneumococcal vaccine has yet been included in Iran’s Expanded Program on Immunization (EPI). However, the most common serotypes circulating in Iran and detected in the present study are similar to those in the European and Asian countries. It appears that the use of pneumococcal vaccines, especially PCV-13, has no role in the prevalence of these serotypes in Iran. Colonizing different bacterial serotypes in Iranian and international tourists’ throats causes the entry of these serotypes to Iran.
Tatochenko et al. (16) investigated the distribution of pneumococcal serotypes and detected 19F, 14, 6B, and 23F as the most common pneumococcal serotypes in Russia. Moreover, 19A was standard in community-acquired pneumonia, acute otitis media, and antibiotic-resistant isolates in all age groups. In the current study, serotypes 6B, 14, 19A, and 1 were the most common serotypes. It appears that the widespread use of PCVs might reduce IPD incidence. Currently, 6B and 14 are the most common serotypes (12, 13).
In the present study, the prevalence of serotype 3 was 6.5%. This serotype was reported only in pneumonia associated with empyema within 1990 – 2001 (17). Unlike other countries, serotypes 4, 7C, and 18C were not detected in the current study. In a study performed by Talebi et al., eight serotypes were identified among the clinical isolates (18), including serotypes 14 (46%), 3 (10.7%), 23F (14.3%), 19F (25%), 19A (0%), 6A (0%), 9V (4%), and 6B (0%). In the present study, serotype 6B was the most prevalent among the studied serotypes. However, serotype 6B had the lowest prevalence in Talebi et al.’s study (18). The significant difference between these two studies regarding serotype prevalence reveals that the currently common serotype among IPD cases was only included among normal flora 6 years ago.
Serotypes 6A, 6B, 14, 19F, and 23F are the most prevalent in children under 2 years of age; however, serotypes 1, 6B, 14, 18C, and 23F are the most common in children of 2 to 5 years (nonpublication). According to studies carried out in the United States, serotypes 6, 14, 18, and 19 are the most common among children under 2 years of age (19). The most common serotypes in Iranian children have been 6B, 14, 19A, and 1, respectively. The prevalence of common serotypes in Iranian children is similar to the prevalence of common serotypes in American children of 2 to 5 years of age (12, 13).
The frequencies of serotypes included in PCV-7 and PCV-10 were 38.2% and 50.7% for clinical isolates, respectively. Currently, pneumococcal vaccines are not included in the EPI of Iran and are only administered to a limited number of individuals who are at high risk of IPDs. The current study’s results suggest that PCV-13 is superior to PCV-7 and PCV-10 to be included in the Iranian National Immunization Program.
Based on the present study’s results, 83% and 84% of the serotypes isolated from the IPD and non-IPD cases comprised vaccine serotypes. The PCV-13 covered 83.5% of all the identified serotypes in this study. Therefore, vaccination with PCV-13 might prevent the prevalence of common serotypes between invasive and noninvasive groups in Iran.
Nonvaccine serotypes detected in this study (16.5%) included 16F (2.3%), 34 (2.3%), 31 (3.5%), 35B (5.9%), and 35F (2.3%). Among nonvaccine serotypes identified in the current study, serotype 35B appeared to be common among French and American nonvaccine serotypes. The studies have reported increases in nonvaccine serotypes, such as 6C, 15A/B/C, 23A, and 35B in the United States (20), 15A, and 23B in Norway (21), and 12F, 15A, 24F, and 35B in France (22).
The introduction of PCV-13 contributed to decreases in IPD (23, 24), pneumonia (including community-acquired pneumonia without bacteremia) (25, 26), and acute otitis media caused by S. pneumoniae belonging to vaccine serotypes, mostly 6A and 19A (27). The current study’s results confirmed that 17.7% of the identified serotypes (i.e., 31, 35B, 34, 16F, and 35F) were not covered by PPSV23.
5.1. Conclusions
In conclusion, the applied multiplex PCR can be a suitable and cost-effective method to identify S. pneumoniae serotypes. It is recommended to use PCV-13 to prevent pneumococcal disease due to the high coverage of PCV-13 among invasive and noninvasive serotypes in Iran. Currently, PCV-13 might be an appropriate vaccine according to the frequency of the current pneumococcal serotypes circulating in Iran.