1. Background
With the emergence and spread of coronavirus disease 2019 (COVID-19) globally, health care systems have faced the biggest challenge in recent decades (1). When we wrote this article, the prevalence of COVID-19 infection was still increasing worldwide. Bacterial or fungal co-infections in COVID-19 patients have been reported around the world. However, risk factors associated with the development of these infections have been poorly studied (2). Most published studies on fungal infections in COVID-19 are related to invasive pulmonary aspergillosis (3-8). Like influenza patients, acute respiratory distress syndrome (ARDS) is a risk factor for the development of Aspergillus infections in hospitalized COVID-19 patients (3). The disease severity, the lack of treatment, use of corticosteroid therapy, and intubation of critical patients are considered risk factors for oropharyngeal candidiasis (OPC) in COVID-19 patients (9). Besides, OPC is the most common opportunistic infection in HIV patients (10). Oral candidiasis has several risk factors in HIV patients, including age, sex, xerostomia, antibiotic usage, alcohol consumption, smoking, CD4 counts, and advanced HIV clinical stage (AIDS) (11). However, Candida spp. as fungal pathogens have been poorly investigated in COVID-19 patients. As known, OPC caused by fluconazole-resistance Candida species can spread systematically in immunocompromised patients through the bloodstream or upper gastrointestinal tract, leading to candidemia with significant morbidity and mortality (12). However, risk factors associated with OPC in COVID-19 patients have not yet been precisely investigated.
2. Objectives
The present study aimed to identify risk factors of OPC in COVID-19 patients in a multi-center evaluation in Tehran, Iran.
3. Methods
3.1. Study Design and Participants
A prospective case-control study was conducted in five COVID-19 clinical centers in Tehran, Iran, between June 1, 2020, and July 1, 2020. In this study, 218 COVID-19 patients were divided into two groups (105 participants as cases and 113 participants as controls). All patients had laboratory-confirmed COVID-19 infection with positive SARS-CoV-2 RT-PCR from nasopharyngeal swabs or respiratory secretions and were hospitalized at Imam Khomeini Hospital Complex, Ziaeian hospital, Valiasr hospital, Labbafinezhad hospital, Emam Ali hospital, and Loghman Hakim hospital. Inclusion criteria were hospitalized adult COVID-19 patients with OPC diagnosed based on clinical presentations and laboratory features. Sampling was done from individual lesions using sterile swabs. The diagnosis was confirmed by the presence of budding yeast and pseudohyphae in KOH 10% preparation and culture. The swabs were streaked on Sabouraud dextrose agar (Difco Laboratories, Detroit, Mich) plates on the bedside. Plates were incubated at 37°C for 48 h and checked morphologically. After identifying Candida species, the isolates were confirmed based on a three-step 21-plex PCR method. Also, the control group was selected randomly from adult COVID-19 patients who had no evidence of OPC during hospitalization. The forms were completed at the time of discharge or death.
3.2. Variables and Data Sources
The questionnaire used in this study consisted of demographic data, treatment strategy, clinical and laboratory features, and underlying diseases. After a questionnaire was completed, it was checked by the expert team to verify the data. The COVID-19 cases that complained of oropharyngeal symptoms were examined, and the patients with a clinically confirmed diagnosis of OPC were included in the case group. Those without clinical features and complaints of OPC on admission were included as the control group. Levels of oxygen support were categorized as a nasal cannula, simple mask, non-invasive ventilation (NIV), mechanical ventilation (MV), or none. Lung involvement in chest CT was identified as less than 25%, 25 - 50%, 50 - 75%, or more than 75%. The variables obtained from the case and control groups were age, sex, hospitalization duration, past medical history, underlying diseases, COVID-19 clinical manifestations, pulmonary involvement, preliminary laboratory tests on admission, type of oxygen support, presence of oral lesions, time of presentation, and recovery period (poor oral hygiene determined by tooth decays, periodontal tissue inflammation, secretion, bleeding or swollen gums, and loose teeth), COVID-19 treatment strategy (pulse therapy methylprednisolone ≥ 250 mg daily, corticosteroids other than pulse: dexamethasone 4 - 8 mg/daily, prednisolone 1 mg /daily/PO, and/or methylprednisolone < 250 mg/daily), antifungal therapy, and four weeks’ outcome.
3.3. Statistical Analysis
All data were analyzed using SPSS 19 (SPSS Iberica, Madrid, Spain). Descriptive analysis was used for demographic and clinical characteristics. Clinical and laboratory data were compared between the case and control groups. Bivariate analysis was performed on all study variables using the Chi-square test. A P value of less than 0.05 was considered significant.
3.4. Ethical Clearance
The study protocol was in line with the principles of the Helsinki Declaration and was approved by the Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.VCR.REC.1399.058). Written informed consent was obtained from all participants.
4. Results
4.1. Characteristics of Patients
A total of 218 COVID-19 patients were included in this study (Imam Khomeini Hospital Complex n = 108 (49.5%; 53 cases and 55 controls), Ziaeian hospital n = 38 (17.4%; 15 cases and 23 controls), Imam Ali hospital n = 23 (10.6%; eight cases and 15 controls), Labbafinezhad hospital n = 9 (4.1%; four cases and five controls), Valiasr hospital n = 10 (4.6%; nine cases and one control), and Loghman Hakim hospital n = 30 (13.8%; 16 cases and 14 controls). The majority of the cases (58.1%) and controls (58.4%) were males. Furthermore, the mean age in the case and control groups was 61.17 ± 14.8 and 55.13 ± 15.1, respectively (P = 0.967). Pseudomembranous candidiasis (77/105, 73.3%) was the most prevalent form of OPC, followed by erythematous candidiasis (22/105, 21%), angular cheilitis (10/105, 9.5%), hyperplastic candidiasis (9/105, 8.6%), median rhomboid glossitis (10/105, 9.5%), denture stomatitis (1/105, 1%), chips in tongue (34/105, 32.4%), and glossitis (29/105, 27.6%).
4.2. Risk Factors Associated with Oral Pharyngeal Candidiasis in COVID-19
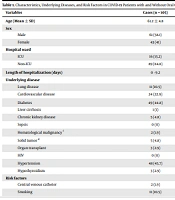
The analysis of variables showed several risk factors statistically significantly associated with OPC in COVID-19 patients (Table 1). Increasing age (P = 0.03), the length of hospitalization (P = 0.016), lung involvement level in chest CT scan (P = 0.001), and diabetes (P = 0.003) were significantly associated with OPC in COVID-19 patients. Medications significantly associated with OPC were chloroquine (P = 0.012), IVIG (P = 0.001), diuretics (P = 0.000), and corticosteroid pulse therapy (P = 0.000). The type of oxygen support was also associated with OPC (P = 0.041). Furthermore, dentures (P = 0.003) and poor oral hygiene (P = 0.000) were related to OPC in the case group (Table 2).
| Variables | Cases (n = 105) | Controls (n = 113) | P Value |
|---|---|---|---|
| Age (Mean ± SD) | 61.2 ± 4.8 | 55.1 ± 5.1 | 0.003 b |
| Sex | 0.967 | ||
| Male | 61 (58.1) | 66 (58.4) | |
| Female | 43 (41) | 46 (40.7) | |
| Hospital ward | 0.343 | ||
| ICU | 16 (15.2) | 14 (12.4) | |
| Non-ICU | 89 (84.8) | 99 (87.6) | |
| Length of hospitalization (days) | 0 - 9.2 | 0 - 7.7 | 0.016 b |
| Underlying disease | |||
| Lung disease | 11 (10.5) | 7 (6.2) | 0.251 |
| Cardiovascular disease | 24 (22.9) | 16 (14.2) | 0.097 |
| Diabetes | 49 (44.8) | 27 (25.7) | 0.003 b |
| Liver cirrhosis | 1 (1) | 1 (0.9) | 0.958 |
| Chronic kidney disease | 5 (4.8) | 6 (5.3) | 0.854 |
| Sepsis | 0 (0) | 1 (0.9) | 0.334 |
| Hematological malignancy c | 2 (1.9) | 2 (1.8) | 0.941 |
| Solid tumor d | 5 (4.8) | 0 (0) | 0.019 b |
| Organ transplant | 3 (2.9) | 1 (0.9) | 0.278 |
| HIV | 0 (0) | 1 (0.9) | 0.334 |
| Hypertension | 48 (45.7) | 26 (23) | 0.000b |
| Hypothyroidism | 3 (2.9) | 6 (5.3) | 0.363 |
| Risk factors | |||
| Central venous catheter | 2 (1.9) | 0 (0) | 0.141 |
| Smoking | 11 (10.5) | 12 (10.6) | 0.973 |
| Antibiotics | 23 (21.9) | 26 (23) | 0.845 |
| Dentures | 25 (23.8) | 10 (8.8) | 0.003 b |
| Poor oral hygiene | 30 (28.6) | 10 (8.8) | 0.000 b |
| Hemodialysis | 1 (1) | 1 (0.9) | 0.958 |
| Foley catheter | 28 (26.7) | 18 (15.9) | 0.052 |
| Mechanical ventilation | 4 (3.8) | 0 (0) | 0.036 b |
| Endotracheal tube | 3 (2.8) | 0 (0) | 0.041 b |
| Corticosteroids | 18 (17.1) | 11 (9.7) | 0.019 b |
| COVID-19 clinical signs | |||
| Fever | 75 (71.4) | 62 (54.9) | 0.011 b |
| Cough | 76 (72.4) | 67 (59.3) | 0.042 b |
| Dyspnea | 82 (78.1) | 80 (70.8) | 0.218 |
| Diarrhea | 12 (11.4) | 19 (16.8) | 0.255 |
| Myalgia | 65 (61.9) | 66 (58.4) | 0.598 |
| Headache | 20 (19) | 25 (22.1) | 0.575 |
| Skin lesions | 1 (1) | 0 (0) | 0.298 |
| Nausea | 17 (16.2) | 25 (22.1) | 0.267 |
Characteristics, Underlying Diseases, and Risk Factors in COVID-19 Patients with and Without Oral Candidiasis a
| Variables | Cases (n = 105) | Controls (n = 113) | P Value |
|---|---|---|---|
| Laboratory results at baseline | |||
| WBC | 10493.9 | 9055.4 | 0.494 |
| Lymphocyte count | 879.1 | 1091.7 | 0.274 |
| HB | 13.2 | 14.5 | 0.374 |
| PLT | 226058.2 | 225657.6 | 0.975 |
| ESR | 65.3 | 63.5 | 0.200 |
| CRP | 54.9 | 58.4 | 0.440 |
| Ferritin | 1107.4 | 572.4 | 0.188 |
| D-Dimer | 559.1 | 400.4 | 0.311 |
| Cr | 1.3 | 1.3 | 0.970 |
| Type of O2 received | 0.041 b | ||
| Mask | 79 (75.2) | 71 (62.8) | |
| Nasal | 14 (13.3) | 24 (21.2) | |
| NIIV | 4 (3.8) | 2 (1.8) | |
| Intubation | 2 (1.9) | 0 (0) | |
| Non | 6 (5.7) | 16 (14.2) | |
| Medication | |||
| Chloroquine | 75 (71.4) | 62 (54.9) | 0.012 b |
| Naproxen | 13 (12.4) | 14 (12.4) | 0.998 |
| Acetaminophen | 14 (13.3) | 21 (18.6) | 0.291 |
| Kaletra | 27 (25.7) | 29 (25.7) | 0.993 |
| Interferon | 63 (60) | 56 (49.6) | 0.122 |
| IVIG | 12 (11.4) | 1 (0.9) | 0.001 b |
| Atazanavir | 63 (60) | 61 (54) | 0.370 |
| Antibiotics | 44 (41.9) | 38 (33.6) | 0.208 |
| Vitamin C | 14 (13.3) | 10 (8.8) | 0.291 |
| Benzodiazepine | 0 (0) | 1 (0.9) | 0.334 |
| Diuretic | 22 (21) | 2 (1.8) | 0.000 b |
| Corticosteroids | 50 (47.6) | 56 (49.6) | 0.775 |
| Corticosteroid pulse therapy | 45 (42.9) | 13 (11.5) | 0.000 b |
| Clinical signs at baseline | |||
| SO2 | 85.16 | 87.26 | 0.019 b |
| RR | 26.10 | 21.81 | 0.039 b |
| PR | 87.37 | 88.38 | 0.716 |
| BP/S | 126.32 | 125.89 | 0.878 |
| BP/D | 73.33 | 75.70 | 0.154 |
| Temperature | 37.38 | 37.12 | 0.185 |
| Outcome after 2 weeks | 0.004b | ||
| Discharge from the hospital | 60 (57.1) | 62 (54.9) | |
| Hospitalized in the ward | 34 (32.3) | 49 (43.3) | |
| Expired | 11 (10.5) | 2 (1.8) | |
| Chest CT | 0.001 b | ||
| Less than 25% | 12 (11.4) | 17 (15) | |
| 25 - 50% | 30 (28.6) | 54 (47.8) | |
| 50 - 75% | 40 (38.1) | 36 (31.9) | |
| More than 75% | 23 (21.9) | 6 (5.3) |
Laboratory and Clinical Data in COVID-19 Patients with and Without Oral Candidiasis by Bivariate Analysis a
4.3. Treatment and Outcome of Oral Candidiasis
Fluconazole (86 patients, 81.9%) and nystatin (40 patients, 38.1%) were considered the antifungal drugs of choice for OPC treatment. In 71 (67.6%) cases, oral lesions were utterly recovered and not recovered in 34 (32.4%) cases at discharge.
5. Discussion
A significant percentage of patients with severe COVID-19 are susceptible to fungal infections such as OPC (1). The treatment of COVID-19 patients with secondary fungal infections is complicated (9). Besides, OPC develops when local host defense is weakened, permitting the fungus to invade and damage oral epithelial cells, such as in HIV-positive patients with several risk factors (13).
Our previous study revealed that several underlying diseases such as cardiovascular and diabetes were more frequent in COVID-19 patients with OPC (9). Therefore, in the present study, we conducted a comparative case-control study for finding risk factors associated with OPC in COVID-19 patients. Numerous factors were analyzed in both case and control groups. We found that several factors were significantly associated with OPC in COVID-19 patients. The age (61.17 ± 14.8 vs. 55.13 ± 15.1, P = 0.03) was a statistically significant risk factor when comparing the case and control groups. A significant reduction in innate salivary defense occurs with aging (14), and several studies showed a relationship between age and OPC in HIV patients (11, 15-17). The length of hospitalization (9.2 days) in the case group had a significant association with OPC in COVID-19 participants (P = 0.016). Patients with a more extended hospital stay may have a higher risk of OPC as they receive more antibiotics and corticosteroids.
The study by Salehi et al. reported that OPC occurs in COVID-19 patients more frequently among cases with eight days’ hospitalization on average (9). In the present study, among the risk factors investigated, dentures (P = 0.003), poor oral hygiene (P = 0.000), and MV (P = 0.036) were statistically significantly related to OPC. Dentures can act as a suitable microenvironment for adherence and overgrowth of the Candida yeast in the mouth, leading to oral candidiasis (18). Endotracheal intubation and MV can cause several complications, such as bacterial and fungal infections (19). Intubation impairs the host's natural defense against infections, and Candida yeast and bacteria can produce biofilms that adhere to the plastic tube (20).
Our findings showed that poor oral hygiene was a risk factor of OPC, in line with previous study (21). Brushing the mouth is essential for cleaning the teeth, dentures, buccal cavity, and tongue. Dentures should be cleaned and left out overnight for at least six hours daily (22). These health measures may be recommended for high-risk COVID-19 patients. In the present study, the lymphocyte count was associated with OPC in COVID-19 patients, and the case group had a lower average lymphocyte count (879.10 cells/mm3) than the control group (1091.73 cells/mm3). A previous study (9) reported lymphopenia in 71.7% of COVID-19 patients with a median lymphocyte count of 1000 cells/mm. A low CD4+ T-lymphocyte count is considered the most important risk factor for developing oral candidiasis in HIV patients (11, 16, 21). We recorded all underlying diseases in both groups. Among them, diabetes (P = 0.003), non-hematological malignancy (P = 0.019), and hypertension (P = 0.000) were statistically significantly associated with OPC in COVID-19 participants. Diabetes decreases the function of the cellular immune system, and these patients are susceptible to opportunistic fungal infections, such as OPC (23).
The finding of a single study performed on the prevalence of OPC in COVID-19 patients showed that diabetes (37.7%) was the major underlying condition (9). Several studies showed that oral candidiasis is a common infection in cancer patients (24-26). This is confirmed by our results in which non-hematological malignancy (P = 0.019) was associated with OPC in COVID-19 patients. Interestingly, hypertension (P = 0.000) had a significant association with OPC in COVID-19 participants in our study. Antihypertensive drugs often cause side effects, such as xerostomia, which is a risk factor for OPC (11, 27). Among medications used for treatment of COVID-19 participants, chloroquine (P = 0.012), IVIG (P = 0.001), diuretic (P = 0.000), and corticosteroid pulse therapy (P = 0.000) were significantly associated with OPC. The use of diuretics can lead to salivary gland hypofunction and xerostomia (11, 28). An increased incidence of oral candidiasis following corticosteroids use has been shown in several studies (29, 30). Some studies showed that chloroquine and IVIG had an antifungal effect on Candida spp. However, in the present study, both factors were significantly associated with OPC in COVID-19 participants (31, 32). Fever (P = 0.011) and tachypnea respiratory rate (P = 0.039) were the most important clinical symptoms associated with OPC. The possible reasons for the high rate of OPC associated with fever and tachypnea can be attributed to dehydration caused by fever and a dry mouth due to the high respiratory rate (19). The main limitations of our study were the lack of mycological examinations, Candida species identification, and their antibiotic susceptibility.
In conclusion, it is reasonable to consider that old age, hospitalization length, poor oral hygiene, corticosteroids use, diabetes, solid tumor, and hypertension may predispose COVID-19 patients to develop OPC. There is a need to strengthen the diagnosis and use effective antifungal and prophylaxis treatment strategies in COVID-19 patients. We concluded that many risk factors and medications could affect the development of OPC in COVID-19 patients. Practical strategies for antifungal prophylaxis may help prevent OPC in high-risk COVID-19 patients.