1. Background
Hydatid cyst is a zoonotic disease caused by the Echinococcus granulosus larvae stage. Its importance is due to the involvement of critical organs, such as the liver and lungs (1). Its prevalence among human populations is as high as 10%, especially in some regions, such as Australia, South Africa, Mediterranean countries, New Zealand, and Asia (2, 3).
Many surgical and non-surgical methods have been suggested for the treatment of hydatid cysts of the liver. For decades, conservative and radical surgical interventions have been the only known treatments for this disease (4). Notwithstanding, the utilization of surgical interventions for the treatment of hydatid cysts has evolved slower than expected due to secondary dissemination and local relapses, especially in conservative surgery (5). Injection of scolicidal agents into the hydatid cyst, during surgery or in the PAIR (puncture, aspiration, injection, re-aspiration) technique, can be used to prevent secondary infection and anaphylactic shock caused by intraperitoneal cyst spillage (6, 7). Until now, several scolicidal agents, such as mannitol, chlorhexidine gluconate (Chx Glu), honey, hypertonic saline, silver nitrate, cetrimide, ethyl alcohol, H2O2, and povidone-iodine, have been used for the inactivation of the hydatid cyst's content (8-15). However, most common scolicidal agents may cause unacceptable side effects, such as secondary sclerosing cholangitis. Therefore, investigations on safe and effective scolicidal agents are currently at the focus of multidisciplinary research initiatives. Despite the large implementation of in vitro studies on the scolicidal agents, due to the need for long-time exposure of these agents to protoscolices, application of them in surgery of hydatid cyst or the PAIR technique is still a challenging surgical issue (16).
Chx-Glu is a widely used mouthwash in dentistry to treat oral infections of the mouths and gums. Chx-Glu affects a wide range of Gram-positive and Gram-negative bacteria as well as some fungi and some viruses, including AIDS-producing viruses and hepatitis. Furthermore, it has a low toxicity effect at therapeutic concentrations of 0.05% in animal models of peritonitis and clinical studies (17-19). Topcu et al. (17) showed the non-toxicity of chlorhexidine gluconate at a concentration of 0.04% on the liver and bile duct tissue by not increasing the level of liver enzymes after its use during hydatid cyst surgery.
2. Objectives
In this experimental animal study, our primary goal was to determine the minimum lethal concentration of chlorhexidine with 100% potency on the protoscolex, and the ultimate goal was to evaluate its side effect on the liver, peritoneum, and biliary duct tissue based on laboratory findings and histological evaluations.
3. Methods
3.1. Preparation of Chlorhexidine Gluconate and Anesthetic Solution
We used the fertile cysts of sheep livers supplied from the local slaughterhouse in Isfahan. The aspirated hydatid liquid was collected into sterile tubes and centrifuged at 1,000 g for 2 minutes, and then the supernatant was discarded. The remaining sediment contained 1500 scolices/ml, which 95 to 99% of that were viable as determined from their motility characteristics are seen with 0.1% eosin staining under light microscopy. The effect of 0.04%, 0.06%, and 0.08% Chx-Glu (GLUCO-Chex, Cerkamed, Poland) during the 5, 10, and 15 minutes on the viability of the protoscolex were evaluated under light microscopy. Anesthesia solution was made with the mixing of nine ccs Ketamine (Ketamine Hydrochloride, Rotexmedica, Germany) and one cc Xylazine 2% (Xylazine Hydrochloride, CASPIAN TAMIN, Gilan, Iran) (20).
3.2. Animal Procedure
3.2.1. Animal Preparation and Ethical Considerations
The experiment was conducted on male New Zealand rabbits (n = 30) that were provided by the experimental animal center of Royan Medical Institute. None of the rabbits had any history of surgery or other medical interventions. All procedures were done according to the guidelines for ethical care of experimental animals and approved by the ethics committee of Isfahan University of Medical Sciences. The animals were kept under controlled conditions in a pathogen-free environment under a constant ambient temperature of 24°C and humidity with free access to food and water. They were allocated into two groups of 15 rabbits (A and B), using a block randomization procedure with matched subjects in each block.
3.2.2. Exclusion Criteria
Impossibility of chlorhexidine injection into the bile duct during the intervention, death of animals before the end of the study for any reason.
3.2.3. Intervention
The procedure for all subjects was carried out under the same standard conditions. After fasting for 4 hours, we intraperitoneally injected 0.1 ccs of the anesthetic solution per 100 grams of rabbit body weight (20). When anesthetization was completed, each rabbit was laid on the surgical table in the supine position, and the abdominal skin was disinfected with 70 % alcohol. Then, blood samples (2 cc) were collected from the tail vein and were sent to a laboratory for assessing the liver enzymes, including alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), and bilirubin (direct and total bilirubin) using a spectrophotometric assay via quantitative diagnostic kits (Parsazmoon Co, Tehran, Iran) in both groups.
In the next step, under the sterile condition and by the guidance of ultrasonography, using the 26 Gage needles, Chx-Glu 0.08% (group A) and Chx-Glu 0.06% (group B) were injected at 1 CC, 2 CC, and 20 CC into the gallbladder, liver parenchyma, and peritoneum cavity, respectively. At the end of the injections, all of the rabbits were kept under the control condition with free access to water and food. Forty-eight hours after injection of Chx-Glu, blood samples (2 cc) were collected again from the tail vein, and liver enzymes were assessed using spectrophotometric assay via quantitative diagnostic kits in both groups. Forty-five days after Chx-Glu injection, after performing the same anesthesia procedure mentioned above, an open biopsy was taken from the peritoneum, gallbladder, and liver parenchyma, and then, histological evaluation was performed by an expert pathologist using hematoxylin-eosin staining on the samples.
3.3. Statistical Analysis
The data were analyzed and reported only for rabbits that completed the study. Data analysis was performed using IBM SPSS software version 24 (Chicago, USA). Data were reported as mean ± standard deviation (SD), and serum ALT, AST, ALP, GGT, and total and direct bilirubin were evaluated. Independent samples t-test was used to determine the statistically significant differences between two groups in terms of continuous variables, and paired samples t-test was used to assess changes in liver enzymes before and after injection in each group. A P-value of less than 0.05 was considered statistically significant.
4. Results
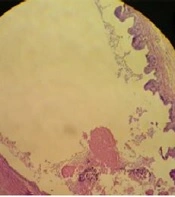
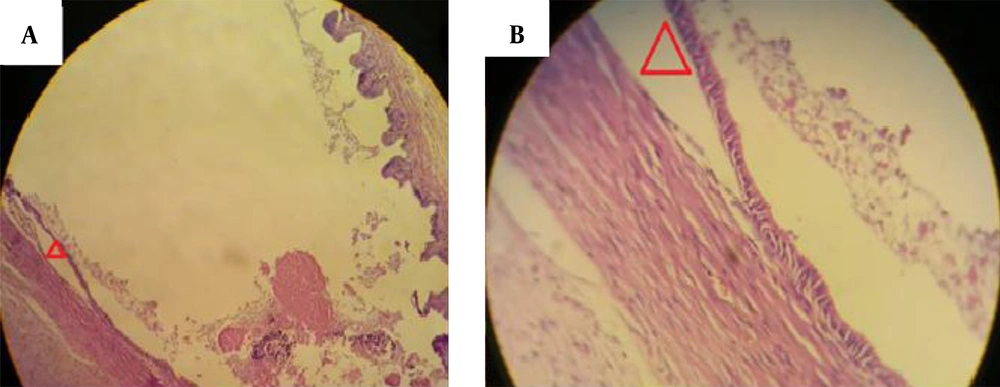
Chlorhexidine gluconate at 0.08% and 0.06% killed 100% of protoscoleces after 5 and 10 minutes, respectively, but Chx-Glu 0.04% could not be effective for inactivating all of the protoscoleces even until 15 minutes (Table 1). Two rabbits in group A and three in group B died one day after the Chx-Glu injection for no apparent reason. In each group before and after the injection of Chx-Glu and between two groups before injection, there were no significant differences (P-value > 0.05) in the mean levels of AST, ALT, ALP, GGT, and direct and total bilirubin. Post-injection mean levels of AST, ALT, ALP, GGT, and direct and total bilirubin were not statistically significantly different between the two groups (P > 0.05) (Table 2). Besides, there was no evidence of significant tissue toxicity (distraction or necrosis) based on histological findings; however, it was revealed that two rabbits in group A and one in group B had focal gall bladder mucosal atrophy with eosinophilic contents that were not histologically significant (Figure 1).
| Chx-Glu Concentration | Percentage of Killed Protoscolex | ||
|---|---|---|---|
| 5 Min | 10 Min | 15 Min | |
| 0.04 | 80 | 80 | 90 |
| 0.06 | 80 | 100 | 100 |
| 0.08 | 100 | 100 | 100 |
Abbreviation: Chx-Glu, chlorhexidine gluconate.
a Values are expressed as %.
| Variables | Pre-test | Post-test | ||
|---|---|---|---|---|
| Group A (Chx-Glu 0.08%) | Group B (Chx-Glu 0.06%) | Group A (Chx-Glu 0.08%) | Group B (Chx-Glu 0.08%) | |
| AST | ||||
| Mean ± SD | 41.53 ± 5.53 | 41.41 ± 9.34 | 44.84 ± 6.66 | 46.08 ± 7.03 |
| P-value | 0.969 | 0.656 | ||
| ALT | ||||
| Mean ± SD | 45.61 ± 11.65 | 43.83 ± 9.36 | 46.23 ± 10.32 | 45.83 ± 11.69 |
| P-value | 0.676 | 0.929 | ||
| ALP | ||||
| Mean ± SD | 100.46 ± 19.88 | 104.33 ± 14.66 | 104.69 ± 16.68 | 105.50 ± 14.31 |
| P-value | 0.583 | 0.898 | ||
| Bill T | ||||
| Mean ± SD | 0.493 ± 0.008 | 0.494 ± 0.005 | 0.496 ± 0.004 | 0.496 ± 0.004 |
| P-value | 0.701 | 0.896 | ||
| Bill D | ||||
| Mean ± SD | 0.195 ± 0.006 | 0.194 ± 0.007 | 0.196 ± 0.004 | 0.195 ± 0.006 |
| P-value | 0.682 | 0.647 | ||
| GGT | ||||
| Mean ± SD | 6.07 ± 1.03 | 5.83 ± 1.19 | 6.38 ± 1.32 | 6.16 ± 1.26 |
| P-value | 0.590 | 0.679 | ||
Abbreviation: Chx-Glu, chlorhexidine gluconate.
5. Discussion
Chlorhexidine is a scolicidal compound that has bactericidal and bacteriostatic properties. Using a scolicidal agent before opening or removing a cyst to inactivate the scolex is strongly suggested (17). To investigate the possible toxicity of Chx-Glu, we evaluated the effect of minimum scolicidal concentration of Chx-Glu on the liver, gallbladder, and peritoneum according to laboratory findings and histological study. It was revealed that there was no evidence of significant tissue destruction or necrosis in the liver parenchyma, gallbladder, and peritoneum 45 days following injections, and also no statistically significant increase in liver enzymes was found 48 hours following injections. Therefore, there were no considerable side effects for Chx-Glu on the peritoneum, gallbladder, and liver parenchyma in animal models.
Bondar et al. showed that peritoneal lavage with Chx-Glu has acceptable efficacy for intraabdominal infections with no toxicity (21). Puryan et al. reported that Chx-Glu 0.04% could be a safe agent for the treatment of intraperitoneal hydatidosis, and 0.04% Chx-Glu solution in a short time (5 min) was effective in both in vitro and in vivo assessments (22). In another similar study, Topcu et al. reported that intra-cystic injection of 0.04% Chx-Glu is an effective agent against the dissemination of viable protoscolices (17). In both studies, authors assessed the efficacy of 0.04% Chx-Glu in hydatid cyst surgery and showed that 5 minutes of exposure to 0.04% Chx-Glu could kill all protoscolices, while the results of our study revealed that this concentration of Chx-Glu could kill only 80% of protoscolices at the same time.
In the present study, 0.06% and 0.08% Chx-Glu solutions had 100% scolicidal efficacy within 10 and 5 minutes, respectively (Table 1), but 0.04% Chx-Glu could not kill all of the protoscolices within 5 and 10 minutes following injection. These differences in the scolicidal potency that was shown in our study may in part be explained by differences of endemic Echinococcus granulosus strains.
Finally, choosing ideal scolicidal agents should depend on some properties, like being potent at low concentrations, acting in a short period, not being affected by dilution with the cyst fluid, and being non-toxic (23). Sclerosing cholangitis is one of the limitations of using the conventional scolicidal agents in the PAIR technique or during the surgery when hydatid cysts are communicated to the biliary tract; thus, Chx-Glu may be useful in these circumstances. Our findings also showed that Chx-Glu at 0.06% concentration was effective and non-toxic, but at higher concentrations, it may have mild toxicity for the gallbladder and biliary tract, according to the histological findings.
5.1. Conclusions
In conclusion, our data suggest that Chx-Glu at 0.08 % and 0.06 % concentrations have 100 % efficacy to kill protoscolices. It seems that Chx-Glu at 0.06 - 0.08 % is an ideal safe scolicidal agent in experimental studies. However, in vivo studies with longer follow-up will be required to establish these findings as clinical evidence.