1. Background
Colorectal cancer (CRC) is one of the most common cancers worldwide (1). It is the leading cause of cancer deaths worldwide and a severe public health issue (2). The CRC risk among different people increases with age (3). Numerous factors such as environmental, genetic, and gut microbiota dysbiosis also have a significant role in developing CRC (4, 5). In general, germs and infectious agents can play a role in the progression of the disease and the severity of malignancies (6, 7). For example, colibactin is the secondary metabolite of Escherichia coli that can disrupt the cell cycle, leading to the growth and spread of CRC (8). Also, oxidative stress, caused by an imbalance between the production of active oxygen radicals and the antioxidant system, can be caused by Enterococcus faecalis and contribute significantly to the progression of cancer (9, 10). Enterococcus faecalis produces reactive oxygen radicals, damaging colon epithelial DNA and being involved in adenomatous polyp's formation and progression into CRC (11).
Some studies on microbial flora and gastrointestinal cancers, especially CRC, show differences in gut microbiota between CRC patients and healthy individuals (12). The essential factors in this difference are age, gut microbiota characteristics, host genotype, changes in the intestinal microbial environment, and nutritional status of the individual (13). Probiotics are living microorganisms (14) that can affect the normal microbial flora and stabilize it, thus having beneficial effects on health (15). Studies have shown that intestinal microbial flora protects against various diseases (16-18). Researchers have attributed these beneficial effects to intestinal microbial balance. Although Lactobacillus and Bifidobacterium are the most commonly used probiotics (19), many microorganisms show such properties (20).
2. Objectives
This study aimed to evaluate the prevalence of E. faecalis, Lactobacillus acidophilus, and Lactobacillus plantarum in people with polyps and CRC compared to healthy individuals in order to find the relationship between these bacteria and CRC and polyps.
3. Methods
3.1. Samples Collection
This study was performed on 60 patients, including 20 healthy individuals, 20 patients with CRC, and 20 patients with polyps. Patients who did not use antibiotics in the previous month, lacked probiotics consumption, had no familial history of intestinal polyps, and had no history of inflammatory bowel disease were enrolled. The selection of individuals to participate in this study was based on colonoscopy and positive pathology results for polyps and CRC and negative pathology results for healthy individuals. Patients' information was recorded in questionnaires. Patients' biopsy samples were placed on ice, collected in appropriate conditions, and transferred to the laboratory.
3.2. Preparation of Standard Strain
We acquired standard strains of E. faecalis (ATCC: 29212), L. acidophilus (DSM20079), and L. plantarum (DSM20174) from the Iranian Biological Resource Center (IBRC). Then, these strains were made on Blood Agar (BA) and Tryptic Soy Broth (TSB) media. After the colonies of bacteria appeared, they were prepared for DNA extraction.
3.3. Genomic DNA Extraction
Genomic DNA was extracted from biopsy specimens and standard strains by the QIAamp DNA biopsy mini kit (Qiagen) and tissue genomic DNA extraction mini kit (Favorgen Biotech Corp). After extraction, NanoDrop 2000 (Thermo Scientific) was used to determine the concentration, and then they were kept at -20°C before the amplification steps.
3.4. Assessment of the presence of Enterococcus faecalis, Lactobacillus acidophilus, and Lactobacillus plantarum by Polymerase Chain Reaction
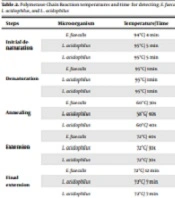
Forward and reverse primers for detecting E. faecalis, L. acidophilus, and L. plantarum strains are shown in Table 1. The PCR temperatures and time for designing our program are shown in Table 2. The PCR products were analyzed by electrophoresis on 2% agarose gel and confirmed by visualizing DNA bands using UV illumination.
Specific Primers for Detecting Enterococcus faecalis, Lactobacillus acidophilus, and Lactobacillus plantarum by Polymerase Chain Reaction
| Steps | Microorganism | Temperature/Time |
|---|---|---|
| Initial denaturation | E. faecalis | 94°C/ 4 min |
| L. acidophilus | 95°C/ 5 min | |
| L. plantarum | 95°C/ 5 min | |
| Denaturation | E. faecalis | 95°C/ 1min |
| L. acidophilus | 95°C/ 1min | |
| L. plantarum | 95°C/ 1min | |
| Annealing | E. faecalis | 60°C/ 30s |
| L. acidophilus | 58°C/ 40s | |
| L. plantarum | 60°C/ 40s | |
| Extension | E. faecalis | 72°C/ 40s |
| L. acidophilus | 72°C/ 30s | |
| L. plantarum | 72°C/ 30s | |
| Final extension | E. faecalis | 72°C/ 12 min |
| L. acidophilus | 72°C/ 7 min | |
| L. plantarum | 72°C/ 7 min |
Polymerase Chain Reaction Temperatures and Time for Detecting Enterococcus faecalis, Lactobacillus acidophilus, and Lactobacillus plantarum
3.5. Assessment of the Presence of Enterococcus faecalis, Lactobacillus acidophilus, and Lactobacillus plantarum by Quantitative Real-time PCR (qRT-PCR)
The qRT-PCR was performed in a reaction volume of 20 µL comprising 10 µL SYBR Green PCR master mix, 0.5 µL of each forward and reverse primers, 2 µL of rox, and 2 µL of DNA extracted from the biopsy specimen. The amount of DNA was determined in duplicate, and the mean values were specified. Amplification and detection of DNA were accomplished by the LightCycler® 96 real-time PCR System (Roche Life Science). The reaction conditions were as follows: 94°C for 3 min, 35 cycles of 95°C for 40 s, 55°C for 25 s and 72°C for 5 min (E. faecalis), 94°C for 2 min, 40 cycles of 94°C for 45 s, 60°C for 25 s and 72°C for 5 min (L. acidophilus), 94°C for 12 min, 35 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 5 min (L. plantarum). Sequence detection software light cycler 96 was used for data analysis. The purified genomic DNA in the range of 1 ng of standard strains was used for determining the amount of E. faecalis, L. acidophilus, and L. plantarum by qRT-PCR. Three different concentrations of standard strains were chosen in the sample editor section. Then, the amounts of copied bacteria in each gram of biopsies were determined by referring to the standard curves.
3.6. Statistical Analysis
Completed questionnaires by patients and the qRT-PCR data were analyzed. Descriptive statistics were conducted using the Statistical Package for Social Sciences (SPSS) software (version 21). Appropriate statistical tests, such as chi-square, Mann-Whitney, and Kruskal-Wallis, were applied depending on the variables. P-values of less than 0.05 were considered statistically significant.
4. Results
The mean age was 50 ± 12.3, 54 ± 17, and 54 ± 8.4 years for healthy individuals, CRC patients, and polyp patients, respectively. There were no statistically significant differences between the groups regarding age and gender (P > 0.05). Five (25.0%) CRC patients, seven (35.0%) polyp patients, and nine (45.0%) healthy individuals had a high-fat diet. There was no statistically significant difference in diet status (high-fat diet) between the groups (P > 0.05). Also, 11 CRC patients, 10 polyp patients, and four healthy people had type O blood. Statistical analysis showed a significant difference in the number of people with type O blood between polyps or CRC groups and healthy individuals (P < 0.05).
The mean copy numbers of E. faecalis, L. acidophilus, and L. plantarum showed a significant difference between the three groups (P < 0.05). The mean copy number of E. faecalis was higher in CRC patients than in polyp patients and healthy individuals (P < 0.05) (Table 3). Also, the mean copy numbers of L. acidophilus and L. plantarum were higher in healthy individuals than in polyp patients and CRC patients (P < 0.05) (Table 3).
| Microorganism | Type of Sample | Minimum | Maximum | Mean |
|---|---|---|---|---|
| E. faecalis | Healthy | 1.4 × 102 | 8.4 × 104 | 2.2 × 103 |
| Polyp | 9.1 × 103 | 7.9 × 105 | 2.3 × 104 | |
| CRC | 3.3 × 106 | 1.9 × 1014 | 9.4 × 1010 | |
| L. acidophilus | Healthy | 9.4 × 1010 | 6.9 × 1013 | 5.0 × 1012 |
| Polyp | 8.6 × 107 | 9.2 × 1010 | 2.9 × 109 | |
| CRC | 4.9 × 107 | 3.9 × 1010 | 9.3 × 108 | |
| L. plantarum | Healthy | 8.9 × 106 | 1.9 × 1014 | 9.7 × 1010 |
| Polyp | 8.6 × 103 | 8.1 × 108 | 2.4 × 105 | |
| CRC | 7.7 × 103 | 9.8 × 104 | 1.3 × 104 |
Copy Numbers of Enterococcus faecalis, Lactobacillus acidophilus, and Lactobacillus plantarum in Biopsy Samples Taken from Patients with Colorectal Cancer, Polyps, and Healthy People
5. Discussion
Colorectal cancer is one of the most common malignancies in men and women worldwide (22). The prevalence of this cancer is significantly increasing in developing countries (23). Some factors are critical in exacerbating or preventing CRC (24). Among these factors are microorganisms, mainly bacteria (25). Therefore, it is essential to discuss the role of these pathogens in CRC.
Gut bacteria are essential in protecting people or causing disease (26). Some bacterial species exacerbate tumor formation and cause cancer, but others maintain intestinal health (27). Various studies show that bacteria can play a role in apoptosis and inflammation in some types of cancer (28-30). Studies also show that some bacteria are involved in chronic infections or the production of toxic agents and can affect cell growth and lead to tumorigenesis (10, 31). According to studies, some bacteria can escape from the immune system. They can also stimulate the immune response through specific cytokines, including interleukin-8 (IL-8) released by inflammatory cells, reactive oxygen species (ROS), and nitric oxide (NO). These substances and other factors such as smoking can significantly increase carcinogenicity. One of the present study results, which was performed by the qRT-PCR method on biopsy specimens, was the higher number of E. faecalis in patients with polyps and CRC than in healthy individuals. Also, CRC patients were found to have a higher number of E. faecalis than those with polyps, and in both groups, it was higher than in healthy individuals. Also, there was a significant difference in the numbers of E. faecalis between CRC patients and polyp patients (P < 0.05). Nevertheless, there was no significant difference in the number of E. faecalis between polyp patients and healthy individuals (P > 0.05). Animal studies have shown that E. faecalis alters the DNA of colon epithelial cells and increases colitis, dysplasia, and adenocarcinoma in mice (32, 33). These study results regarding this bacterium's role in exacerbating or developing CRC confirm the current study findings. Animal studies also showed that changes in the immune system and inflammatory responses could play an essential role in cancer progression.
The metabolic activity of E. faecalis in the gastrointestinal tract causes it to produce extracellular superoxide (O2), leading to colon polyps or CRC. Thus, E. faecalis can play an important role in exacerbating gastrointestinal cancer. There is evidence that treatment with probiotics can modulate the gastrointestinal function and reduce gastrointestinal disorders (34, 35). Consumption of probiotics can lead to the production of fermented products such as short-chain fatty acids. Probiotics induce the colon's protective enzyme glutathione transferase II (36). These factors reduce the genetic material load in the gut and increase the production of agents inactivating toxic compounds. Butyrate, for example, is one of these protective compounds slowing cancer cell growth (37). Studies also show that the abundance of bacteria and bifidobacteria reduces the risk of colon polyps (38, 39). However, there is ample evidence that probiotics, especially lactobacilli, play an influential role in fighting cancer. They affect the digestive enzymes of animals and humans, inhibit carcinogens in vitro and in vivo, and suppress compounds that induce cancer and tumors (40).
In this study, the SYBR Green method evaluated the copy number of L. acidophilus and L. plantarum in biopsy specimens. According to the results, the mean copy number of L. acidophilus was higher in healthy individuals than in polyp and CRC patients (P < 0.05). Also, this bacterium's mean copy number was higher in polyp patients than in CRC patients. However, there was no significant difference in the mean copy number of L. plantarum between the polyp and CRC groups. There are several reasons to explain these findings. For example, L. acidophilus, one of the most critical and common bacteria in gut flora, may be altered by changes in the gut due to polyps and CRC. Also, L. plantarum is one of the most abundant species of the Lactobacillus genus, commonly found in fermented foods. As a result, the extended antimicrobial activity of L. plantarum is one of the most important therapeutic factors for preventing infections. The present study results on the copy number of L. acidophilus in biopsy specimens obtained from polyp and CRC patients compared to healthy individuals are similar to those obtained from L. plantarum. A study determined the role of Lactobacillus in inducing or preventing the spread of adenoma or CRC (41). Based on the results, lactobacilli can be essential in inducing or spreading malignancy. The results of this study and our previous study show the dual role of bacteria in preventing or inducing malignancy. These results could pave the way for extensive study of the microbiome of CRC patients compared to healthy individuals. These studies can also provide insight into how bacteria affect health and disease.
Research has shown that the population of some bacteria, including Fusobacterium nucleatum, is higher in patients with CRC than in healthy individuals (42). This increase may be due to a decrease in the population of bacteria such as L. acidophilus and L. plantarum, which in turn leads to dysbiosis. In 2004, Darfeuille-Michaud et al. (43) showed that CRC increased the population of E. coli in the colon mucosa. In 2014, Zackular et al. (44) found that Fusobacterium or bacteria of the Fusobacteriaceae family were more common in the fecal samples of patients with CRC than in healthy individuals. However, the population of intestinal Proteobacteria decreases in patients with CRC. The researchers showed that the population of bacteria such as E. coli, Citrobacter, Shigella, Flavobacterium, Acinetobacter, and Chryseobacterium was reduced in patients with CRC (45). The findings of Baldwin et al. also suggest that L. acidophilus and L. casei may increase the induction of apoptosis in the carcinoma cell line and may be used as adjuvant chemotherapy (46). Zhang et al. showed that lactic acid bacteria bind to the Trp-P-1 mutagen and reduce the absorption of this substance in the gut, thus reducing the risk of cancer (26). Goldin et al. showed that L. casei has the ability to reduce the activity of three harmful fecal enzymes, beta-glucuronidase, nitroreductase, and azoreductase (47). These enzymes can convert pre-mutagenic and precancerous compounds into mutagenic and carcinogenic compounds. Chiu et al. also showed that soluble compounds secreted by L. casei induce apoptosis in monocytic leukemia cells (48). As a result, probiotics as a supplement can effectively prevent polyps from turning into cancer. Ultimately, there was a significant difference in type O blood between polyp and CRC patients and healthy individuals. Therefore, people with type O blood may be more prone to polyps and CRC.
5.1. Conclusions
In light of the results obtained in this study and the results acquired from previous studies, physicians can prescribe probiotics to help prevent CRC after adenomatous polyps are detected. Probiotics in the diet of the elderly can prevent the development of polyps or their progression to malignancy. In this study, there was a significant difference in blood types. Type O blood was more prevalent than other blood groups in polyp and CRC patients. Therefore, it can be claimed that CRC is more common in people with blood type O, which can be prevented using a proper diet and probiotics.