1. Introduction
Strongyloides stercoralis infection is of great importance because, due to its cycle of self-inoculation, it is the only worm that can remain in the gastrointestinal tract without clinical manifestations for decades (1, 2) The infection can reactivate in some transplant recipients after starting immunosuppressive therapy and might manifest clinically with dry cough, hemoptysis, dyspnea, duodenitis, and diarrhea (1). The patient can also present with severe disease, manifesting either as hyperinfection syndrome, an extension of the normal life cycle of the nematode, leading to an excessive burden of worms in the traditional reproductive pathway, or as a disseminated disease, defined by the presence of parasites outside the traditional life cycle (i.e., in organs other than the skin, gastrointestinal tract, or lungs) (2). There is limited available information about severe strongyloidiasis in transplant recipients (3).
The most recommended treatment for intestinal strongyloidiasis is oral ivermectin. In immunocompromised hosts, to prevent the occurrence of hyperinfection or disseminated disease, ivermectin (200 µg/kg/day) for 2 consecutive days and repeated for the same period in the second week is recommended (4) There is no parenteral anthelmintic presentation licensed for use in humans; therefore, the appropriate dose, pharmacokinetics, and safety of their use are unknown (5). In disseminated strongyloidiasis, the mortality rate is almost 80%. As a result, early diagnosis and treatment are essential to increase the chance of recovery from the infection (2). Herein, the authors report a case of disseminated strongyloidiasis in a renal transplant recipient treated with parenteral Ivermectin complicated with neurological manifestations.
2. Case Presentation
A 66-year-old male patient with a hepatorenal polycystic disease, nephrolithiasis, and prostatectomy due to prostate cancer received a kidney transplant from a 25-year-old male deceased donor (cause of brain death: traumatic brain injury and cold ischemia time of 27 hours and 30 minutes). The pre-transplant dialysis vintage was 3 years. Induction therapy consisted of thymoglobulin, and maintenance therapy included everolimus and tacrolimus without prednisone. The initial clinical evolution was satisfactory, with good renal function and no acute rejection. The case was discharged 7 days after transplant with a serum creatinine 1.2 mg/dL. Albendazole 400 mg for 5 days and a single dose of secnidazole 2.0 g were prescribed as an antiparasitic preventive measure.
Thirty-five days after transplantation, the patient complained of adynamia, asthenia, hyporexia, epigastralgia, abdominal cramps, and black stools. The patient was admitted on the next day, presenting nausea, vomiting, and dehydration. Upper endoscopy showed intense erosive duodenitis. The polymerase chain reaction for cytomegalovirus and polyomavirus was negative for both viruses. The patient was discharged on the third day of hospitalization with the prescription of pantoprazole for duodenitis and ivermectin as an additional preventive measure for the parasitic disease. It was difficult to ensure the patient adequately followed the prescription at home.
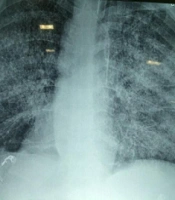
Three days after discharge, the patient was readmitted to the hospital due to persistent epigastric pain and emesis. Treatment with ganciclovir, teicoplanin, and meropenem was started empirically. The following day, the patient developed a dry cough and respiratory distress. A chest X-ray showed diffuse interstitial infiltrates (Figure 1).
A high-resolution chest computed tomography revealed a nonspecific bilateral micronodular infiltrate. A bronchoalveolar lavage (BAL) bronchoscopy demonstrated a bilateral normal-looking bronchial mucosa and the presence of blood-stained secretion coming from the left lower lobe. In BAL, S. stercoralis larvae and Klebsiella pneumoniae were detected. A biopsy from the duodenum also revealed a larval form of S. stercoralis in mucosal crypts. The patient was admitted to the intensive care unit (ICU). He was hemodynamically stable and on the venturi mask. Meropenem was maintained, and the remaining antimicrobials and immunosuppressive drugs were discontinued. Empirical therapy with caspofungin, azithromycin, and ivermectin was added. The latter drug was taken at a dosage of 200 μg/kg/day orally for 5 days.
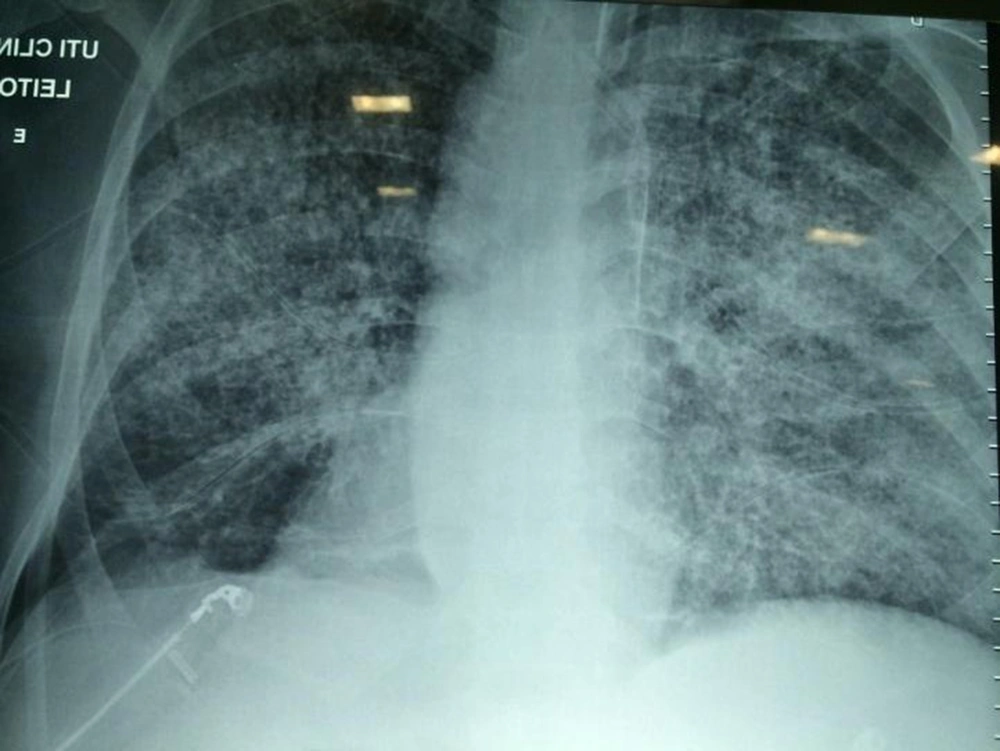
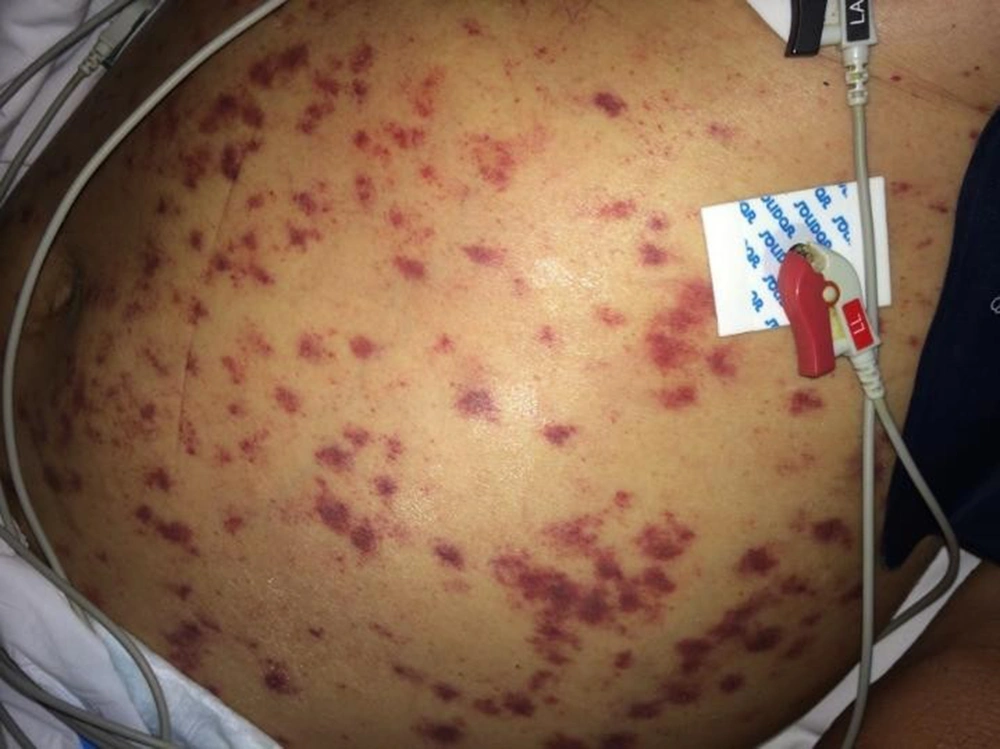
On the third day of intensive care, the patient developed respiratory failure, disorientation, and abdominal petechiae (Figure 2), requiring mechanical ventilation, in addition to vasoactive drugs, with suspected sepsis. A chest X-ray showed a worsening of the pulmonary infiltrate (Figure 2). On the fifth day of intensive care, hemodialysis therapy was indicated.
Disseminated petechial lesions associated with acute respiratory distress syndrome corroborated the diagnosis of disseminated strongyloidiasis. As there was no clinical improvement with oral treatment, a parenteral veterinary formulation of ivermectin (0.2 mg/kg/day) was initiated for 4 days.
During hospitalization, the patient had a failure to wake up after cessation of sedation, and a skull computed tomography was normal. After 13 days in the ICU, the patient did not verbalize and presented an eye-opening reflex. A flaccid tetraparesis was detected, with muscle strength grade 0 in the upper extremities and grade 1 in the lower ones.
The reflexes were diffusely hypoactive, with a bilateral absent plantar reflex and an automatic withdrawal of a limb from a painful stimulus. A lumbar puncture was performed, demonstrating elevated protein levels (141.0 mg/dL) and normal cell counts (1.0/mm3) in cerebrospinal fluid (CSF). Five days later, another CSF analysis confirmed the aforementioned results. The patient died after 31 days of hospitalization due to the failure of multiple organs
3. Discussion
Strongyloidiasis is a parasitic disease with a mortality rate of around 50% in cases of hyperinfection and might increase to 87% in the disseminated form (6). Transplant patients 8 are at risk for developing severe strongyloidiasis due to immunosuppressive therapy used to prevent rejection. It has been reported that the risk of strongyloidiasis dissemination increases after organ transplantations, such as the heart, liver, pancreas, intestine, and, more frequently, kidney, even in non-endemic countries (3).
The potent immunosuppressive drug tacrolimus is a calcineurin inhibitor that reduces interleukin-2 (IL-2) production, IL-2 receptor expression, and T lymphocyte activity. The transplant recipient is more likely to become infected with Strongyloides, and once infected, it is more likely to develop severe cases of strongyloidiasis due to an increase in the reproductive cycle of the larvae that could migrate to various other organs (6). On the other hand, cyclosporine, which was the most commonly prescribed calcineurin inhibitor prior to the advent of tacrolimus, might reduce this risk as it has direct antiparasitic activity, protecting against hyperinfection syndrome (7). In the clinical case in question, the patient used tacrolimus, which contributes to such early hyperinfection.
For the diagnosis of disseminated strongyloidiasis, a high degree of clinical suspicion is required since clinical signs are nonspecific. Unexplained gastrointestinal or pulmonary symptoms in susceptible patients should be warning signs. The most frequent manifestations are abdominal pain, acute respiratory distress, cough, hemoptysis, hypoxemia, and shock. Sepsis might occur due to Gram-negative germs, as Strongyloides facilitate the translocation of enterobacteria through the intestinal mucosa. Skin involvement in disseminated strongyloidiasis is a rare and potentially fatal manifestation. The presence of these lesions might represent a valuable sign for diagnosis, a fact observed in the reported patient.
Regarding the definitive laboratory diagnosis of disseminated strongyloidiasis, it is necessary to find larvae in the stool, tracheal secretion, bronchial lavage, gastric aspirate, or gastric, jejunal, dermal, and pulmonary biopsies (2). In the present case, S. stercoralis larvae were observed in BAL and duodenal biopsy.
Screening patients for asymptomatic Strongyloides infection is crucial to prevent hyperinfection syndrome. The methods for the diagnosis of the parasitic infection are stool examination, molecular diagnostics, and serologies, with the latter being both the most reliable and sensitive, especially in endemic areas. Patients at high risk for dissemination with a potential Strongyloides-exposure history should be screened, especially those with diseases associated with the risk for hyperinfection syndrome and those requiring immunosuppressive therapy (8). If the infection is detected, the treatment must be initiated immediately.
For the treatment of severe strongyloidiasis, the oral administration of ivermectin is the treatment of choice, with a cure rate of 100%, compared to thiabendazole, which has a cure rate of 78%. ivermectin is also much more tolerated and is associated with greater eradication of parasite larvae when compared to albendazole and has fewer side effects than thiabendazole. Moreover, according to the literature, ivermectin toxicity is low, and the administration of 200 μg/kg/day for 1 - 2 days promotes the best efficiency and tolerability relation (4). In the present study, albendazole was used as the preventive measure for parasitic diseases, with inadequate prevention of strongyloidiasis, and the patient evolved to hyperinfection syndrome.
The treatment of disseminated strongyloidiasis should be started immediately after clinical suspicion. If the clinical status of a patient receiving oral therapy does not improve during treatment, the measurement of serum ivermectin levels or replacement of the oral route by the parenteral one is recommended. Serum ivermectin levels within the range of 11.4 - 49.6 ng/dL have been correlated with microscopic evidence of parasite eradication (2).
In this context, Chiodini et al. showed that multiple subcutaneous doses of ivermectin, which have significantly higher plasma levels, compared to the oral route, are well-tolerated with no side effects in patients with suspected Strongyloides hyperinfection (9). Salluh et al. also used subcutaneous ivermectin (0.2 mg/kg/day) after patient consent, which resulted in clinical improvement, no side effects, and discharge from the patient after three doses of medication (10). In the case of this report, parasitic damage to intestinal lymphatic vessels and intestinal mucosa, with subsequent edema of the intestinal wall, probably contributed to reducing the absorption of the oral anthelmintic, corroborating the use of parenteral ivermectin. In Brazil, there are no licensed parenteral anthelmintic drugs for human use. Given the severity of the case, a veterinary subcutaneous formulation was initiated with the consent of the patient’s family. Even in other countries, ivermectin is only approved for oral administration (11).
Toxic effects of oral ivermectin on the central nervous system (CNS), especially mydriasis and ataxia, have already been described; however, the use of ivermectin was well tolerated and showed no indicators of CNS toxicities up to 10 times higher than the recommended dose by the Food and Drug Administration (200 µg/kg). The mydriasis effects between the treated and placebo groups were similar at all tested concentrations (12). Confusion, ataxia, drowsiness, encephalopathy, and coma have been related to the side effects of the parenteral drug. Additionally, plasma ivermectin levels are significantly higher with subcutaneous administration, compared to the oral route, which might account for the higher survival and cure rates of patients treated with parenteral ivermectin (13).
In the present case, the patient presented with a neurological manifestation, with a clinical suspicion of critical illness polyneuropathy or Guillain-Barré syndrome (GBS). Critical illness polyneuropathy is prevalent among ICU patients exposed to risk factors, including sepsis (14). It is a sensory-motor axonal neuropathy that leads to muscle weakness. Muscle weakness usually predominates in the proximal parts of the limbs and is usually symmetrical. Decreased or absent deep tendon reflexes might be present, and sensory loss is detected in some patients (15). Electroneuromyography is the gold standard method to define the diagnosis. It determines an axonal polyneuropathy pattern, ruling out the possibility of spinal cord injury. In these cases, CSF examination is normal or mildly altered, and nerve biopsy shows primary axonal degeneration without evidence of inflammation (14).
The GBS is an acute or subacute demyelinating polyneuroradiculopathy characterized by symmetrical and bilateral flaccid limb paralysis, deep areflexia, mild sensory changes, and CSF albumin-cytological dissociation, often preceded by respiratory or gastrointestinal infections (16). Distal paresthesia increases the likelihood that the correct diagnosis is GBS. Albumin-cytological dissociation is present in about 50% of GBS patients during the first week of the disease, although this number increases to 75% in the third week (17).
Although the association of GBS with disseminated strongyloidiasis has not been previously reported in the literature, the neurological manifestations of the present case associated with the finding of albumin-cytological dissociation on CSF analysis suggested this complication. As precipitating factors for GBS, several hypotheses were made, including the use of parenteral ivermectin, S. stercoralis hyperinfection, or other unknown triggering factors. However, diagnostic confirmation with electroneuromyography or nerve conduction studies was not possible.
In the case of parenteral ivermectin administration, the drug might promote mydriasis, ataxia, tremor, vomiting, lethargy, and coma (9, 13). Since such symptoms were not observed in the present case, it is unlikely that ivermectin was a precipitating factor for GBS. In addition, it has been observed in the literature that very low albumin levels result in the increased clearance of ivermectin and, consequently, lower serum concentrations of the drug (18). Serum albumin levels of 1.1 g/dL were detected in the present case, which could have resulted in lower blood concentrations.
Regarding the parasite as a triggering factor for GBS, high migration of the larvae into the body might invade the peritoneum, liver, lung, and CNS in hyperinfection syndrome (19). Recently, GBS has been reported after coronavirus disease 2019 and Zika virus infection (20).
Therefore, immunosuppressed patients with severe epigastric pain and vomiting must have strongyloidiasis included in their differential diagnosis, and early treatment is essential for minimizing the risk of disseminated disease. It is also relevant to consider the early use of parenteral ivermectin in its most severe clinical presentation. However, in view of the present report, parenteral ivermectin should be used with caution, preferably with the measurement of blood levels, in addition to monitoring CNS toxic symptoms. It is also required to be aware that S. stercoralis infection in its disseminated form might be a triggering factor for GBS.