1. Background
After more than one year of its emergence, COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is still ongoing, leading to over 80 million confirmed cases and about two million deaths (1), yet no signs of the pandemic remission exist. The lack of curative treatment and vaccines, along with new manifestations, has made challenges in disease management. Positive real-time reverse transcriptase-polymerase chain reaction (RT-PCR) test results in patients recovering from clinical symptoms with documented negative RT-PCR several days to months later are one of these unknown novel phenomena during the pandemic.
2. Objectives
We determined clinical and laboratory characteristics of Iranian COVID-19 patients who returned with symptoms compatible with COVID-19 and positive RT-PCR for SARS-CoV-2 after recovery from the first episode. Moreover, we reviewed the literature and discussed possible explanations.
3. Methods
We retrospectively evaluated the results of SARS CoV-2 PCR tests in the laboratory registries of three main hospitals (Imam Khomeini Hospital Complex, Masih Daneshvari Hospital, and Imam Hussein Hospital Medical Center) in Tehran during the first six months of the pandemic. All patients who had the following criteria were included in the study: Two positive tests three months apart, a negative PCR test between the two positive tests, and access to the patient and medical information in both episodes. Then, we interviewed all eligible patients and recorded the patients' clinical, laboratory, and radiological characteristics in data collection forms. We also compared the clinical and laboratory characteristics in the first and second episodes.
Two reviewers evaluated the data separately. The mild, moderate, and severe diseases were defined according to WHO guidelines (2). Based on the national recommendations, the nasopharynx swab sample collection and detection of SARS-CoV-2 and COVID-19 pneumonia definition were performed.
This study was approved by the Ethics Committee of Amir Alam Hospital, Tehran University of Medical Sciences, with the ethics code of IR.TUMS.VCR.REC.1399.088. Informed consent was obtained from all participants.
Statistical analysis was performed using SPSS, version 22.0. Quantitative and qualitative data were reported as mean ± SD and number (%), respectively. Continuous and categorical variables were compared using the independent t-test and the χ2 test, respectively. The P-values less than 0.05 were considered statistically significant.
4. Results
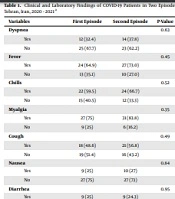
A total of 32,567 tests were performed. Besides, 1,899 patients were tested three times, among whom 138 patients had two positive tests. However, 37 cases were included in the study based on our criteria. Clinical and laboratory characteristics of 37 COVID-19 patients in the first and second episodes are shown in Table 1. Among these patients, 24 (64.9 %) persons were males. The mean age was 37.54 ± 15.16 years (24 to 94 years). The majority of them were nurses (37.8%) and other Healthcare Workers (HCW) (35.1%). All cases were immunocompetent. Seven (18.9%) patients had cardiovascular diseases, and two (5.4%) had diabetes. The mean body mass index (BMI) was 25.84 ± 3.25. The CT scans showed pneumonia in 56.8% of the cases in the first episode. Weakness (75.7%), myalgia (73.0%), and fever (64.9%) were the most frequent clinical symptoms in the first visit. On the first exposure, the mean O2 saturation (SO2) was 92.9 ± 4.07. Only two (5.4%) patients had severe disease, two (5.4%) were hospitalized, and no one died. In addition, lymphopenia was observed in four (33.3%) cases. Considering treatment, 13 (35.1%) patients received antiviral therapy, and dexamethasone was administered only for one (2.7%) person in the first episode.
Comparing the two episodes, there were no significant differences between clinical and laboratory characteristics of 37 COVID-19 patients, except for dexamethasone use (P = 0.03) (Table 1). The mean interval between the two episodes was 117 ± 61.42 days.
| Variables | First Episode | Second Episode | P-Value |
|---|---|---|---|
| Dyspnea | 0.63 | ||
| Yes | 12 (32.4) | 14 (37.8) | |
| No | 25 (67.7) | 23 (62.2) | |
| Fever | 0.45 | ||
| Yes | 24 (64.9) | 27 (73.0) | |
| No | 13 (35.1) | 10 (27.0) | |
| Chills | 0.52 | ||
| Yes | 22 (59.5) | 24 (66.7) | |
| No | 15 (40.5) | 12 (33.3) | |
| Myalgia | 0.35 | ||
| Yes | 27 (75) | 31 (83.8) | |
| No | 9 (25) | 6 (16.2) | |
| Cough | 0.49 | ||
| Yes | 18 (48.6) | 21 (56.8) | |
| No | 19 (51.4) | 16 (43.2) | |
| Nausea | 0.84 | ||
| Yes | 9 (25) | 10 (27) | |
| No | 27 (75) | 27 (73) | |
| Diarrhea | 0.95 | ||
| Yes | 9 (25) | 9 (24.3) | |
| No | 27 (75) | 28 (75.7) | |
| Weakness | 0.39 | ||
| Yes | 28 (75.7) | 31 (83.8) | |
| No | 9 (24.3) | 6 (16.2) | |
| Antiviral therapy | > 0.9 | ||
| Yes | 13 (35.1) | 13 (35.1) | |
| No | 24 (64.9) | 24 (64.9) | |
| Taking Steroid | 0.03 | ||
| Yes | 1 (2.7) | 8 (21.6) | |
| No | 36 (97.3) | 29 (78.4) | |
| Outcome | > 0.9 | ||
| Death | 0 (0) | 1 (3.1) | |
| Discharge | 32 (100) | 31 (96.9) | |
| Disease level | 0.12 | ||
| Mild | 24 (64.9) | 19 (51.4) | |
| Moderate | 11 (29.7) | 10 (27) | |
| severe | 2 (5.4) | 8 (21.6) | |
| Hospitalization | 0.15 | ||
| Yes | 2 (5.4) | 7 (18.9) | |
| No | 35 (94.6) | 30 (81.1) | |
| O2 Saturation | 92.9 ± 4.1 | 90.1 ± 7.3 | 0.13 |
| C-reactive protein (CRP) (mg/L) | 23.6 ± 32.7 | 28.1 ± 30.3 | 0.73 |
| White blood cell (WBC) (cell/mcL) | 7049.2 ± 1889.2 | 7595 ± 2862.5 | 0.55 |
| Lymphocyte count (cell/mcL) | 1401.4 ± 793.3 | 2093.9 ± 977.5 | 0.05 |
| Chest CT scan | 0.25 | ||
| Positive | 21 (56.8) | 16 (43.2) | |
| Negative | 16 (43.2) | 21 (56.8) |
a Values are expressed as No. (%) or mean ± SD.
A CT scan showed ground-glass opacities suggesting active infection in 43.2% of cases at the second exposure. Weakness (83.8%), myalgia (83.8%), and fever (73%) were the most frequent clinical symptoms. The mean SO2 was 90.1 ± 7.3. Eight (21.6%) patients had severe disease, seven (18.9%) were hospitalized, and one (2.7%) died. Lymphopenia was observed in two (12.5%) cases. Considering treatment in this episode, 13 (35.1%) patients received antiviral therapy, and dexamethasone was administered for eight (21.6%) persons (Table 1).
5. Discussion
In this study, most of the cases were males, and the most predisposing factor was cardiovascular disease. Weakness, myalgia, and fever were the most frequent clinical symptoms in each episode. Pneumonia was detected in nearly half of the patients in both episodes.
Except for significantly more dexamethasone consumption in the second episode, the clinical and laboratory characteristics were not significantly different between the two episodes. Growing evidence in favor of dexamethasone use could explain this finding. Severe disease was only observed in less than one-third of all episodes.
Ye et al. reported similar clinical characteristics in patients with the second episode of COVID-19. Fatigue, fever, and cough were the most frequently reported symptoms, in sequence, and none of them had dyspnea or severe pneumonia. In contrast, all elderly re-admitted COVID-19 patients died, as reported by Lafaie et al. They also remained asymptomatic between the two episodes (3, 4). Li et al. reported chest pain and cough as re-presenting symptoms, whereas fever and hypoxia were reported in another patient who was re-admitted because of COVID-19. Both of them survived, although the latter referred to an inpatient rehabilitation facility (5, 6). At least four case series reported lymphopenia and elevated concentrations of CRP in most re-admitted patients (3, 4, 7, 8). However, in line with our series, Wang and Li reported normal lymphocyte count and CRP level in the majority of re-admitted patients (9, 10).
Among potential host risk factors for the second episode (gender, older age, and taking immunosuppressive agents) mentioned by Ye et al., our patients were mainly male and had cardiovascular disease. Older age and interleukins suppression mediated by steroids may play a significant role, as six out of 16 (38%) patients in the previous series (3, 7) and all the older patients in Lafaie’s study received corticosteroids in the first episode (3, 4). However, our patients were relatively younger.
The mean interval of 117 days between the two episodes in our study is longer than 4 to 17 days reported in previous studies (10, 11). However, Lafaie et al. reported a period of more than 30 days for a dead old patient (4).
Considering chest CT features of re-admitted patients, in line with our series, Li et al. showed a changing pattern from reticulation to ground-glass opacity (GGO), indicating active infection, which occurred in 40% of patients (10). However, most re-hospitalized patients in the Zheng series had improved CT abnormalities (12). Several scenarios have been proposed to explain this phenomenon, including persistent infection, reinfection, relapse or reactivation, and inflammatory rebound.
5.1. Persistent Infection
The long clinical course of COVID-19 more than two months has been documented (13). In addition, continuous viral shedding irrespective of clinical symptoms was reported as long as 83 days by Li et al. (14). The average range of viral shedding marked by positive RT-PCR was noted 20 to 22 days after symptom onset (6). On the other hand, the false‐negative rate of RT‐PCR results was 12.5% in one study (15). It could be due to low viral loads, collecting specimens by different methods, and laboratory errors (10). Therefore, prolonged viral clearance or persistent infection rather “turning positive again” or “reoccurring” has been proposed (16). Moreover, Wang et al. supported the transmission of the whole virus or traces of the virus from the lower respiratory tract to the throat or nose as their patients had only slight coughs (9). However, our patients and Ye, Lafaie, and Gousseff series remained asymptomatic between the two episodes and presented again with notable symptoms such as fever and hypoxia, making this scenario doubtful (3, 4, 7). Besides, the re-emergence of GGO in at least 40% of re-admitted patients in the Li et al. series provides another clue against persistent infection (10). Finally, culturing the virus in the second episode showed the cytopathic effect of SARS-CoV-2, as Gousseff et al. did for two patients (7), demonstrated the true; however, we could not perform it. Moreover, the RT-PCR cycle threshold (Ct) value below 24 may correlate with viable SARS-CoV-2, which was unavailable in our RT-PCR results (17).
5.2. Reinfection
As a rule, circulating antibodies, memory B cells, and memory T cells are three essential parts of the immune system to prevent viral reinfection. The presence of SARS-CoV-2-specific T cells documented in COVID-19 could quarantine the immunity of recovered patients even in the absence of specific antibodies; however, potential anamnestic B and T cell responses remain obscure (18).
Since no recurrent disease was observed after re-challenging the monkeys with the same strain of SARS-CoV-2, Duggan et al. concluded that different strains could be responsible for reinfection (6). Unfortunately, we could not perform the phylogenic sequencing in the returning patients to evaluate this claim.
Since four HCW of the Gousseff series had mild symptoms in both episodes, and persistent exposure could be expected, they suggested reinfection might occur (7). In contrast, based on the Ravioli et al. series, reinfection was less likely given that the prevalence of COVID-19 was low in that region (8). Most recently, To et al. documented reinfection in an immunocompetent patient by phylogenetic analysis of the virus in two episodes, challenging herd immunity or even vaccination (19).
5.3. Relapse or Reactivation
While a shorter interval between the two episodes was in favor of reactivation (4), the mean interval in our cases was more than 110 days. Suboptimal control of SARS-CoV-2 infection or the presence of sanctuaries was purposed as the causes of relapse in these patients (7). In the first episode, underlying host conditions, SARS-CoV-2 viral load, and immunosuppression state might predispose the patients to reactivation (3). It seems that old patients in the Lafaie et al. series had a reactivation episode given the less likelihood of re-exposure and relatively short interval between the two episodes; also, two patients had negative serology at re-admission (4). Seven older patients with comorbidities and a median of 11 symptom-free days, as reported by Gousseff et al., appeared to have a similar scenario (7).
In line with the Gousseff series, our patients had mainly mild disease and were healthcare personnel. In addition, the prevalence of COVID-19 was high during the study period, and the mean interval between the two episodes was nearly four months. Therefore, reinfection is probably the best scenario justifying our case series.
5.4. An Inflammatory Rebound
Dysregulated immune reaction might be responsible for clinical deterioration, but the virus successfully cultured in re-admitted patients of the previous series makes this hypothesis less likely (7). All of our patients also had positive RT-PCR for SARS-CoV-2 in the second episode.
5.5. Another Diagnosis
Recurrence of clinical symptoms could be due to other pneumonia-causing bacteria and viruses or secondary complications such as pulmonary embolism; however, nearly all differential diagnoses of COVID-19 in Lafaie et al.’s series and ours had been ruled out (4).
We investigated only three COVID-19 referral hospitals retrospectively. The virus culture, phylogenic sequencing of SARS-CoV-2, and the RT-PCR cycle threshold were not determined. Therefore, multi-state prospective studies performing the mentioned exams are warranted.
5.6. Conclusions
Although we could not cultivate the virus and perform the phylogenic sequencing of SARS-CoV-2 in the re-presenting patients with positive RT-PCR for SARS-CoV-2 and symptoms compatible with COVID-19, the prolonged interval between the two episodes suggests probable reinfection in our cases. Given the substantial impact on public health and vaccination outcomes, the investigation of the precise physiopathology of this phenomenon is urgently warranted. Finally, this clinical phenomenon may be more common in HCW without a significant consequence; however, most cases were HCW who were more compatible with our criteria due to the availability of their medical information.