1. Background
Breast cancer is one of the most common health problems. It is also the prevalent cause of death in women. Several factors can develop breast cancer, such as obesity, family history, estrogen levels, estrogen receptors, adipokines, leptin and adiponectin, exogenous and endogenous modulators of oxidative stress, and viruses (1).
Herpesviruses, with double-stranded DNA, cause human and animal diseases. Although most herpesvirus members show differences in tissue tropism and mechanism of interaction with their host, the DNA replication process is highly conserved during infection (2). Herpes simplex virus type 1 and type 2 (HSV-1 and HSV-2) are members of Alphaherpesvirinae, which are very common in the human population and cause different clinical manifestations after infection (3). HSV-1, one of the most common human viruses, is usually passed on by oral contact during childhood and adolescence. However, HSV-2 is more common in sexually active adults and adolescents and transmitted through sexual intercourse (1, 4). The global prevalence of HSV-1 is estimated to be close to 67%, and that of HSV-2 is 11 - 20% (3). The genomic sequence homology of these two viruses is reported to be about 50% (5). These viruses, with relatively large envelopes and a double-stranded linear genome (152 kb in length and 90 RNA transcripts), require 4 to 12 hours for the cell proliferation cycle and usually lead to cell death (except for some neurons) or cause latent infections in the cells (5).
Oncological viruses, such as herpes viruses (HSV-1), are also used in cancer treatment strategies because they replicate to kill tumors without harming healthy cells (6). However, the sensitivity of cells to HSV-1 cytotoxicity is varied. For example, bone marrow hematopoietic cells are resistant, while breast cancer cells are sensitive (7). HSV-1 is a proper therapeutic choice for several reasons, such as its large genome, abundant non-essential proteins, ease of manipulation, natural oncolytic properties, and broad tissue tropism. However, viral proteins can express it 3 to 6 hours after infection, such as infected cell protein 4 (ICP4), ICP27, ICP34,5, Us3, and Us5 (gJ). These proteins block the apoptosis process and allow the virus to multiply efficiently (8). HSV-2 is also effective in treating both primary and metastatic breast cancer (9).
2. Objectives
This study aimed to evaluate the frequency of HSV-1 and HSV-2 viral genomes and the levels of HSV-IgM and HSV-IgG antibodies in the serum of patients compared to healthy subjects to determine the relationship between these viruses and breast cancer.
3. Methods
The Ethics Committee of Shahid Beheshti University of Medical Sciences reviewed and approved the study protocol. The survey was conducted following the guidelines of Helsinki's Declaration. Under the supervision of a specialist, 60 healthy women (40 with fibroadenoma and 20 in good health) and 60 women with breast cancer who needed surgery from those admitted to Tehran hospitals were selected. Their cancer was confirmed by mammography, sonography, and biochemical tests. The healthy subjects were in the same age range as the patients, and they and their first-degree relatives had no underlying diseases also signs of developing cancer. Informed consent was obtained from all the participants, and basic information was available for further analysis of the results.
3.1. DNA Extraction and Real-time PCR
After surgery, a portion of the breast tissue was placed in a cryotube by a pathologist to extract all the tissue DNA using a commercial kit (Invitrogen; Thermo Fisher Scientific Inc, USA). After confirming the quality and quantity of the extracted samples, they were amplified with specific primers of the beta-globin gene as the housekeeping gene (F: 5' GAAGAGCCAAGGACAGGTAC3' and R: 5'CAACTTCATCCACGTTCACC 3'). Specific primers for DNA polymerase were designed to identify viruses, which were used for proliferation by real-time PCR. HSV-1 (Gene Bank: X04771/1) (Forward: 5' AACAAGGAGGAGGTCGACAG 3' and Reverse: 5' GAAGTTGTCGCACAGGTACG 3') and HSV-2 genes (Gene Bank: M16321/1) (Forward: 5' AGATCAAGGTGAACGGGATG 3' and Reverse: 5' GCGGCAGAAACTTGAAGAAC 3'). Amplification reactions were performed in volume 20 µL (100 ng DNA, 10 µL master mix (SYBRTM Green 2X qPCR master mix), and 1 µL of each primer with 7 µL of deionized distilled water) under the following conditions: 95°C for 10 min, 35 cycles at 95°C for 1 min, 60°C for 30 s, and 72°C for 30 s using the ABI 7500 real-time PCR (applied biosystems, life technologies). Samples containing HSV-1 and HSV-2 viral genomes were used as positive controls (taken from the Keivan Virology Laboratory), and DNA-free samples were used as negative controls. All the reactions were duplicated.
3.2. Serology
Serum was collected from all the subjects 24 h before the operation and stored at -80°C until the test was performed. After collecting all the samples, the serum concentration of IgM and IgG antibodies against HSV was evaluated with a commercial kit (Immunolab GmbH, Germany) by the ELISA technique. The findings were compared with the results of real-time PCR. The kit OD cutoff value was higher than 10 U/mL. According to the kit protocol, the concentration of antibodies was measured against the kit standards.
3.3. Statically Analysis
The results were statistically analyzed using IBM SPSS software version 23. The chi-square and Fisher’s exact tests were used to statistically compare the positive tests for HSV-1 and HSV-2 between cancer and healthy groups. The age average, body mass index (BMI), and IgM and IgG antibody levels were measured in cancer and healthy groups using an unpaired t-test. All analysis results were reported as mean ± SD, and the P-value < 0.05 was considered significant.
4. Results
Women in both groups were in the age range of 41 to 64 years, and the mean age was significantly different between the cancer patients (55.30 ± 7.79) and the healthy subjects (46.97 ± 6.47) (P = 3.73 × 10-9). However, no significant difference was observed between the BMI of the two groups (24.35 ± 18.87 kg/m2 in the case group and 24.79 ± 2.66 kg/m2 in the control group.
The HSV-1 genome was detected in six cancer patients and two healthy cases (P = 0.143). Regarding HSV-2, three cancer patients and one healthy subject were positive (P = 0.309). Tables 1 and 2 provide the demographic information and positive real-time PCR results distribution for the HSV-1 and HSV-2 genomes.
| Variables | Number | HSV-1 Genome | P-Value | |
|---|---|---|---|---|
| Pos | Neg | |||
| Age | 0.526 | |||
| > 50 | ||||
| Case | 44 | 5 | 39 | |
| Control | 18 | 0 | 18 | |
| ≤ 50 | ||||
| Case | 16 | 1 | 15 | |
| Control | 42 | 2 | 40 | |
| BMI | 0.279 | |||
| ≤ 25 | ||||
| Case | 39 | 4 | 35 | |
| Control | 29 | 2 | 27 | |
| > 25 | ||||
| Case | 21 | 2 | 19 | |
| Control | 31 | 0 | 31 | |
| ER (Case) | 0.911 | |||
| Pos | 49 | 5 | 44 | |
| Neg | 11 | 1 | 10 | |
| PR (Case) | 0.417 | |||
| Pos | 39 | 3 | 36 | |
| Neg | 21 | 3 | 18 | |
| Type of cancer | 0.334 | |||
| Invasive lobular carcinoma (ILC) | 16 | 0 | 16 | |
| Invasive ductal carcinoma (IDC) | 32 | 5 | 27 | |
| Ductal carcinoma in situ (DCIS) | 8 | 1 | 7 | |
| Lobular carcinoma in situ (LCIS) | 4 | 0 | 4 | |
| Stage of cancer | 0.684 | |||
| I | 5 | 0 | 5 | |
| II | 11 | 2 | 9 | |
| III | 19 | 2 | 17 | |
| IV | 25 | 2 | 23 | |
| Variables | Number | HSV-2 Genome | P-Value | |
|---|---|---|---|---|
| Pos | Neg | |||
| Age | 0.342 | |||
| > 50 | ||||
| Case | 44 | 3 | 41 | |
| Control | 18 | 0 | 18 | |
| ≤ 50 | ||||
| Case | 16 | 0 | 16 | |
| Control | 42 | 1 | 41 | |
| BMI | 0.194 | |||
| ≤ 25 | ||||
| Case | 39 | 1 | 38 | |
| Control | 29 | 0 | 29 | |
| > 25 | ||||
| Case | 21 | 2 | 19 | |
| Control | 31 | 1 | 30 | |
| ER (Case) | 0.40 | |||
| Pos | 49 | 3 | 46 | |
| Neg | 11 | 0 | 11 | |
| PR (Case) | 0.192 | |||
| Pos | 39 | 3 | 36 | |
| Neg | 21 | 0 | 21 | |
| Type of cancer | 0.192 | |||
| Invasive lobular carcinoma (ILC) | 16 | 0 | 16 | |
| Invasive ductal carcinoma (IDC) | 32 | 2 | 30 | |
| Ductal carcinoma in situ (DCIS) | 8 | 0 | 8 | |
| Lobular carcinoma in situ (LCIS) | 4 | 1 | 3 | |
| Stage of cancer | 0.725 | |||
| I | 5 | 0 | 5 | |
| II | 11 | 0 | 11 | |
| III | 19 | 1 | 18 | |
| IV | 25 | 2 | 23 | |
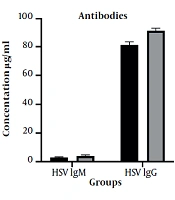
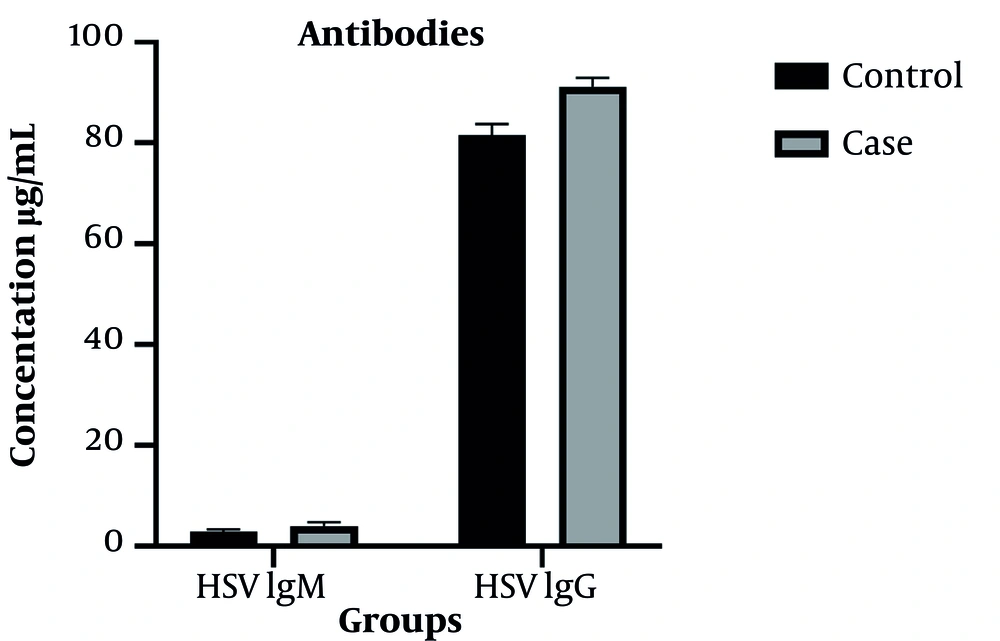
The HSV IgM antibody was positive in six cancer patients and three healthy subjects (P = 0.298). However, there was no statistically significant difference between the cancer group (4.01 ± 5.91 U/mL) and the healthy group (2.95 ± 3.51 U/mL) in terms of the mean serum concentration of HSV IgM antibody (P = 0.236). Figure 1 compares the serum concentration of antibodies in cancer and healthy groups.
The HSV IgG antibody was positive in the serum of all cancer and healthy subjects. However, there was a statistically significant difference between cancer (91.22 ± 13.58 U/mL) and healthy groups (81.58 ± 17.02 U/mL) regarding the mean serum concentrations of HSV IgG antibody (P = 0.001).
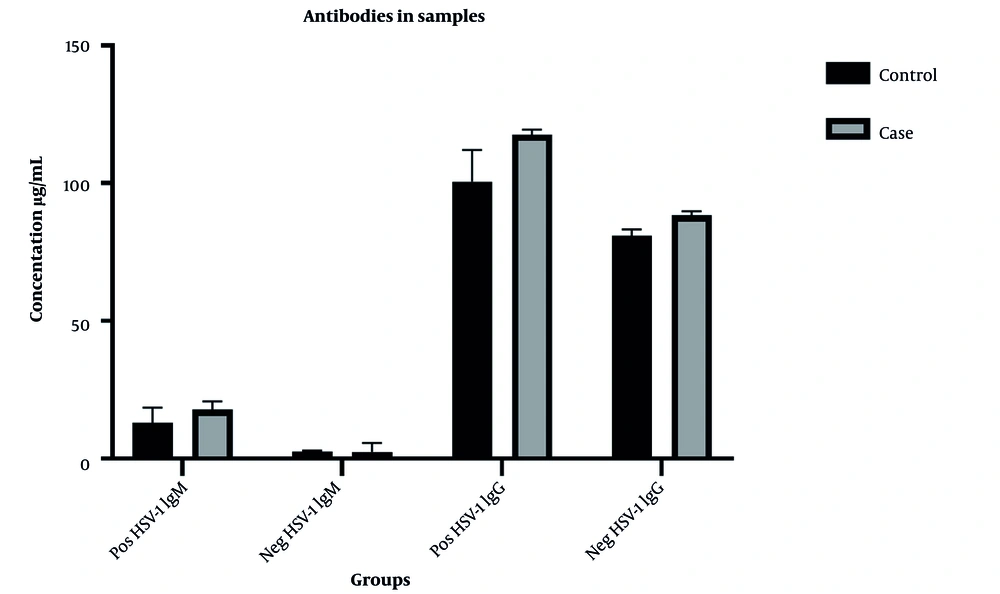
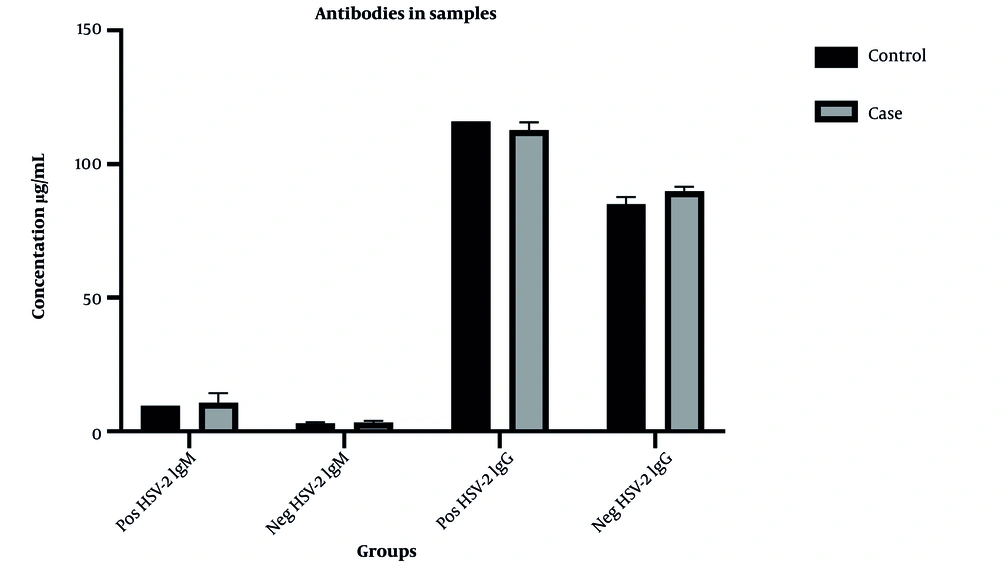
Serum concentrations of the antibodies in the positive and negative samples for HSV-1 & HSV-2 are shown in Figures 2 and 3, respectively.
5. Discussion
One of the most common malignancies identified in the majority of women in the world is breast cancer (1). Certain viruses, such as herpesvirus, polyomavirus, papillomavirus, and retrovirus, can cause breast cancer. They probably play a role in carcinogenesis by different mechanisms, including co-factor activity in NF-κB, STAT3, and HIF1α pathways (10). Various studies on viruses and breast cancer have shown conflicting results. However, some DNA viruses are more common, such as human papillomavirus, Epstein-Barr virus, human cytomegalovirus, herpes simplex virus, and the human herpes virus type 8 (1).
In the present study, HSV-1 was observed in six cancer patients and two healthy subjects (P = 0.143). Also, HSV-2 was observed in three cases in the cancer group and one in the healthy group (P = 0.309). Tsai et al. indicated that HSV-1 was present in eight cancer samples (out of 69 individuals with breast cancer), and in contrast to this study, HSV-2 was not detected in any of the samples. They examined the presence of six viral genomes and identified more than one viral genome in breast cancer and fibroadenoma samples (11).
Khashma also detected HSV-1 in 31.8% of cancer cases (out of 22 individuals) using the immunofluorescence method (12). In another study, Tsai et al. examined six potent oncogenic viruses, including HSV-1, concerning nodal status and treatment outcome in breast cancer. Although there was no significant association between viruses and breast cancer, HSV-1and CMV viruses were relevant to the overall survival rate (13).
Perhaps, one of the reasons for the absence of the HSV-1 viral genome in tumors is the rapid death of infected cells by apoptosis, which limits the replication of the virus. This virus expresses both apoptosis-inducing and anti-apoptotic genes, and the balance between them determines the mortality rate of infected cells (8).
In this study, HSV-2 was observed in three cancer patients and one fibroadenoma subject (P = 0.309). In the survey by Kaveh et al., HSV-2 was observed by multiplex PCR method in three out of 60 patients with breast cancer (14). In contrast to these studies, Hsu et al. reported no association between HSV-2 and breast cancer (1). Most studies have focused on the role of HSV-2 in uterine cancer development. In the study by Yang et al., out of 27 cervical cancer samples, only one sample was infected with three viruses (HPVtype35, CMV, and HSV-2), and the results did not indicate that HSV-2 could play a direct role in carcinogenesis (15). However, Hildesheim et al. reported that HSV-2 could increase the risk of uterine neoplasms development (16). Interestingly, in subsequent studies, HSV-1 has been proposed as a major cause of genital infections in specific populations due to its increasing prevalence. Most studies have shown that previous immunity against HSV-1 may reduce the asymptomatic infection of HSV-2 (17).
Commercial kits were used to identify antibodies produced against HSV-1 and HSV-2 and evaluate the serum level of the antibodies. Based on the results of the ELISA technique, only six cancer patients and three healthy individuals had the HSV IgM antibody in their serum, and the mean serum levels were not statistically significant. However, the levels were higher in the cancer group than in the healthy group. The HSV IgG antibody was detected in both groups and had a significantly higher mean serum level in the cancer group than in the healthy group (P = 0.001).
Several factors, such as mutations in genes, environmental changes, and also changes in the immune system, play a role in the development and spread of breast cancer. The role of viruses in breast cancer has not been proven yet, and various studies have indicated contradictory results. However, it can be concluded that viruses are directly effective in carcinogenesis by affecting cell-deforming agents, or they, as co-factors, stimulate cell deformation. Generally, some pivotal factors, such as genetic changes, immune system disorders, and viral infections, are necessary for breast cancer development.