1. Background
COVID-19 caused a global pandemic and a new major public health concern in the world (1), with various morbidity (2), mortality (3), and economic effects (4). Therefore, many countries developed COVID-19 surveillance systems (5) to detect patients and subsequently control the pandemic.
COVID-19 infections are categorized into two spectrum manifestations: Asymptomatic and clinical manifestations (6). Although there is an extensive spectrum of clinical symptoms, including mild disease of the upper respiratory system, severe viral pneumonia with an acute respiratory syndrome, and death (7), in general, the clinical symptoms of COVID-19 are unspecific (8). Previous studies (9) showed various clinical manifestations, including fever, chills, cough, breathing difficulty, fatigue, myalgia, irritability or confusion, sore throat, coryza, diarrhea, nausea or vomiting, headache, chest pain, abdominal pain, arthralgia, and anorexia. These symptoms can help diagnose possible infection.
Polymerase chain reaction by real-time reverse transcriptase (RT-PCR) is considered the gold standard for diagnosing COVID-19 infection (10). However, there are limited laboratories for molecular testing (11), and delayed diagnosis makes it challenging to control transmission and provide timely health care. Therefore, clinical manifestations may help the early diagnosis of the disease.
The Communicable Disease Center of the Ministry of Health in Iran collects information about the clinical features of patients with COVID-19 hospitalized in different parts of Iran. The collected information is based on the World Health Organization (WHO) case definition.
2. Objectives
This study aimed to evaluate and compare the diagnostic value of the COVID-19 clinical symptoms. The symptom patterns were extracted by latent class analysis (LCA), and the diagnostic value of the extracted patterns for diagnosing COVID-19 infection was determined.
3. Methods
3.1. Data Sources
The data used in this cross-sectional study was part of the COVID-19 surveillance system, managed by the Centers for Disease Control and Prevention at the Ministry of Health and Medical Education of Iran. The data was collected from all hospitals admitting patients with confirmed or suspected COVID-19 in Mazandaran Province, northern Iran. A total of 13,724 patients were recruited between February 20 and August 20, 2020. The patients were hospitalized through direct visit, referral from outpatient clinics (health care service centers), and referral from other hospitals not dedicated to COVID-19 patients. The name of cities and the number of patients recruited from each city are shown in Appendix 1.
The gold standard was at least one positive RT-PCR result from a specimen collected with a nasopharyngeal swab in verified laboratories and according to the WHO protocols.
Demographic data (age and gender) and clinical symptoms were obtained by interviewing the patients or their caregivers. The clinical symptoms included fever, chills, cough, shortness of breath, fatigue, myalgia, irritability or confusion, sore throat, coryza, diarrhea, nausea or vomiting, headache, chest pain, abdominal pain, and arthralgia.
The study was approved by the Shahid Beheshti University of Medical Sciences (Ethic Code: IR.SBMU.PHNS.REC.1399.097). Informed consent was obtained from all the participations, and all methods were performed under the relevant guidelines and regulations.
3.2. Data Analysis
The analysis was conducted in three steps. First, the relative frequency of the clinical symptoms was compared between patients with and without confirmed COVID-19 infection using chi-square test. Then, the sensitivity (Se) and specificity (Sp) of each symptom were calculated. Finally, the significant clinical symptoms of the previous step were considered for LCA.
Latent class analysis is a person-oriented approach that is similar to cluster analysis. In this study, exploratory LCA focused on classifying patients according to the co-occurrence of multiple clinical symptoms and defined individuals with similar co-occurrence symptoms as a latent class (or a symptoms pattern).
Substantive theory and fit statistics (12), including Akaike's information criterion (AIC), the Bayesian information criterion (BIC), and the sample-size adjusted Bayesian information criterion (a-BIC), were considered to determine the optimal number of classes. Smaller values of AIC, BIC, and aBIC indicate a better model fit (13, 14). Also, the Lo-Mendell-Rubin likelihood ratio test, Vuong-Lo-Mendell-Rubin likelihood ratio test, and the parametric bootstrapped likelihood ratio test were used. A significant P-value in these tests indicates that the k class model is preferred over the k-1 class model (15). In addition, the entropy values ranging from 0 to 1 were determined. A higher entropy value shows a better model fit and a clear separation of classes in values above 0.80 (16). Also, Se and Sp and the area under the receiver operating characteristic (ROC) curve (AUC) for each extracted class (pattern) of LCA were compared and plotted. Area under the receiver operating characteristic curve is a summary measure of Se and Sp and is considered excellent if its values are between 0.9 - 0.99, good if between 0.80 - 0.89, acceptable if between 0.70 - 0.79, and poor if between 0.51 - 0.69 (17).
In the final step, multiple logistic regression was used to determine the odds ratio of each pattern of symptoms by adjusting for gender and age groups for predicting COVID-19 infection. The predicted values by this model were used to estimate AUC.
4. Results
Of the 13,905 patients, 181 (1.3%) were excluded because of missing or unknown RT-PCR test results. The remaining 13,724 patients were included in the analyses. Of the participants, 48.1% were female, and the mean age of the patients was 53.6 ± 20.3. Reverse transcription polymerase chain reaction confirmed COVID-19 infection in 4,836 (35.2%) of the patients (Table 1). The distribution of the clinical symptoms among positive- and negative-COVID-19 patients is displayed in Table 1.
| Symptoms | Total | COVID-19 + | COVID-19 - | χ2 | P-Value |
|---|---|---|---|---|---|
| Fever and chills | 6,346 (46.24) | 2,739 (56.64) | 3,607 (40.58) | 324.74 | < 0.001 |
| Cough | 6,156 (44.86) | 2,631 (54.4) | 3,525 (39.66) | 275.25 | < 0.001 |
| Shortness of breath | 6,944 (50.6) | 2,776 (57.4) | 4,168 (46.89) | 138.35 | < 0.001 |
| Fatigue | 2,034 (14.82) | 773 (15.98) | 1,261 (14.19) | 8.01 | 0.005 |
| Myalgia | 3,242 (23.62) | 1,311 (27.11) | 1,931 (21.73) | 50.30 | < 0.001 |
| Irritability or confusion | 1,002 (7.3) | 302 (6.24) | 700 (7.88) | 12.30 | < 0.001 |
| Sore throat | 2,275 (16.58) | 896 (18.53) | 1,379 (15.52) | 20.55 | < 0.001 |
| Coryza | 382 (2.78) | 152 (3.14) | 230 (2.59) | 3.56 | 0.59 |
| Diarrhea | 601 (4.38) | 267 (5.52) | 334 (3.76) | 23.25 | < 0.001 |
| Nausea or vomiting | 1,203 (8.77) | 470 (9.72) | 733 (8.25) | 8.48 | 0.004 |
| Headache | 1,433 (10.44) | 642 (13.28) | 791 (8.9) | 64.12 | < 0.001 |
| Chest pain | 1,122 (8.18) | 409 (8.46) | 713 (8.02) | 0.79 | 0.374 |
| Abdominal pain | 205 (1.49) | 69 (1.43) | 136 (1.53) | 0.22 | 0.633 |
| Arthralgia | 1,049 (7.64) | 405 (8.37) | 644 (7.25) | 5.65 | 0.017 |
| Other symptoms a | 2,440 (17.78) | 967 (20.0) | 1,473 (16.57) | 25.10 | < 0.001 |
The Distribution of the Clinical Symptoms and Signs in Hospitalized Patients According to Positive and Negative COVID-19 Infection
Symptoms, including fever, chills, cough, shortness of breath, fatigue, myalgia, sore throat, diarrhea, nausea or vomiting, headache, and arthralgia, were significantly more common in patients positive for COVID-19 than in other patients. However, irritability or confusion was significantly more in negative-COVID-19 patients than in their positive-COVID-19 counterparts. Shortness of breath, fever, chills, and cough were common in all the patients.
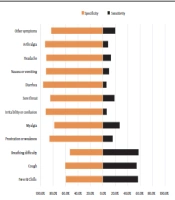
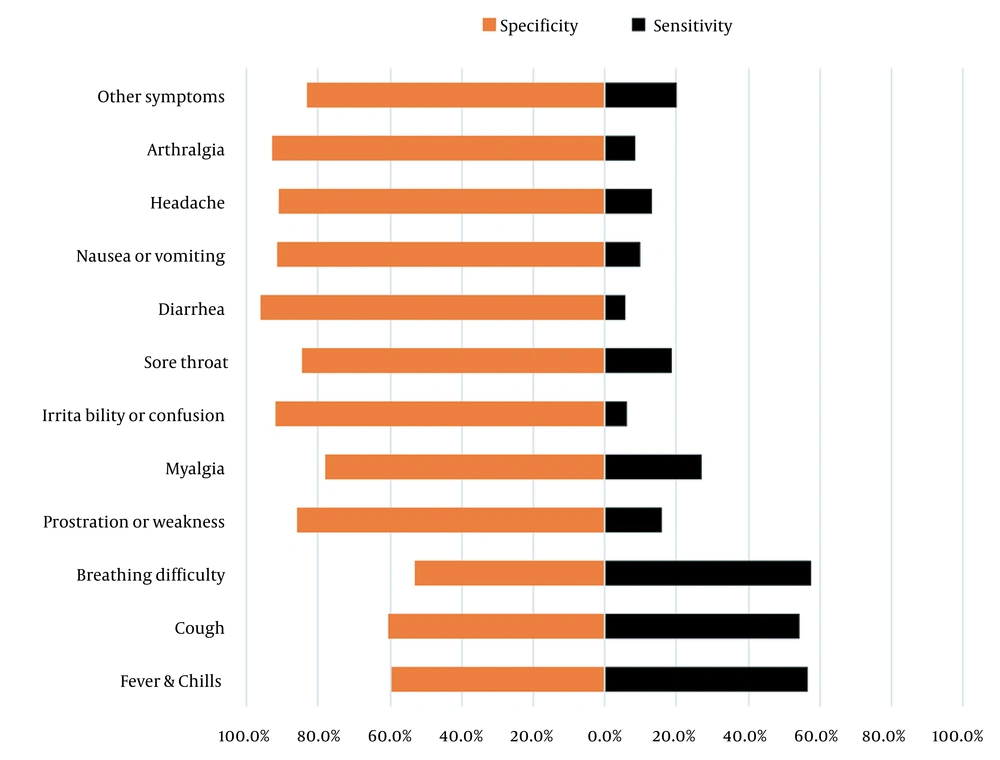
The Se and Sp of each symptom are presented in Figure 1. Shortness of breath (57.4%), fever/chills (56.6%), and cough (54.4%) had the highest Se, while diarrhea (5.5%) and irritability or confusion (6.2%) had the lowest Se.
Two to seven latent class models were run on significant clinical symptoms (Table 2). According to model fit statistics in combination with interpretability, the optimal number of classes for the clinical symptoms was six. Table 3 shows the prevalence of symptom patterns (classes) and the conditional probability of each symptom in the same class (i.e., the existing probability of each symptom in the same class).
| AIC | BIC | aBIC | VLMR | LMR | Entropy | |
|---|---|---|---|---|---|---|
| Class 2 | 140556.4 | 140744.5 | 140665.1 | 4901.1 a | 4861.9 a | 0.87 |
| Class 3 | 139560.4 | 139846.4 | 139725.6 | 1022.1 a | 1013.8 a | 0.56 |
| Class 4 | 139052.2 | 139436.1 | 139274.0 | 534.1 a | 529.8 a | 0.58 |
| Class 5 | 138699.15 | 139180.9 | 138977.5 | 379.0 b | 376.0 b | 0.53 |
| Class 6 | 138467.0 | 139046.6 | 138801.9 | 258.1 c | 256.1 c | 0.57 |
| Class 7 | 138324.4 | 139101.9 | 138815.8 | 168.5 | 167.1 | 0.52 |
Latent Class Analysis of the Fit Indices for the Signs
| Symptoms | Probability of a Yes Response for Each Symptom in the Same Class a | |||||
|---|---|---|---|---|---|---|
| Class 1 | Class 2 | Class 3 | Class 4 | Class 5 | Class 6 | |
| Prevalence | 594 (4.3) b | 1409 (10.3) b | 5500 (40.1) b | 1067 (7.8) b | 3666 (26.7) b | 1488 (10.8) b |
| Fever and chills | 0.86 c | 0.52 c | 0.57 c | 0.52 c | 0.22 | 0.44 |
| Cough | 0.83 c | 0.91 c | 0.55 c | 0.24 | 0.13 | 0.35 |
| Shortness of breath | 0.75 c | 0.77 c | 0.49 | 0.23 | 0.47 | 0.48 |
| Fatigue | 0.24 | 0.25 | 0.02 | 0.18 | 0.14 | 0.31 |
| Myalgia | 0.98 c | 0.15 | 0.12 | 0.16 | 0.02 | 0.99 c |
| Irritability or confusion | 0.14 | 0.05 | < 0.01 | 0.11 | 0.13 | 0.10 |
| Sore throat | 0.54 c | 0.23 | 0.22 | 0.09 | 0.02 | 0.19 |
| Diarrhea | 0.16 | 0.03 | < 0.01 | 0.21 | < 0.01 | 0.04 |
| Nausea or vomiting | 0.28 | 0.06 | < 0.01 | 0.37 | 0.04 | 0.09 |
| Headache | 0.42 | 0.16 | 0.04 | 0.17 | 0.04 | 0.19 |
| Arthralgia | 0.60 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 0.47 |
| Other symptoms | 0.35 | 0.29 | < 0.01 | 0.28 | 0.21 | 0.25 |
Six Latent Class Prevalences of the Clinical Symptoms and the Conditional Probability of Each Symptom in the Same Class
In the LCA of the clinical symptoms, class 1, with a prevalence of 4.3% (n = 594), was characterized by a high probability (probabilities > 0.5) of fever, chills, cough, shortness of breath, myalgia, sore throat, and arthralgia in individuals clustered in this class. Class 2, with a prevalence of 10.3% (n = 1409), had symptom patterns, including fever/chills (probability = 0.52), cough (probability = 0.91), and shortness of breath (probability = 0.77). Class 3, with a prevalence of 40.1% (n = 5500), was characterized by fever/chills (probability = 0.57) and cough (probability = 0.55). Class 4, with a prevalence of 7.8% (n = 1067), was characterized by only fever and chills (probability = 0.52). Class 5, with a prevalence of 26.7% (n = 3666), was characterized by low probability (probabilities < 0.5) for all the symptoms; in other words, patients of this class had no symptoms. Finally, class 6, with a prevalence of 10.8% (n = 1067), was characterized only by myalgia (probability = 0.99).
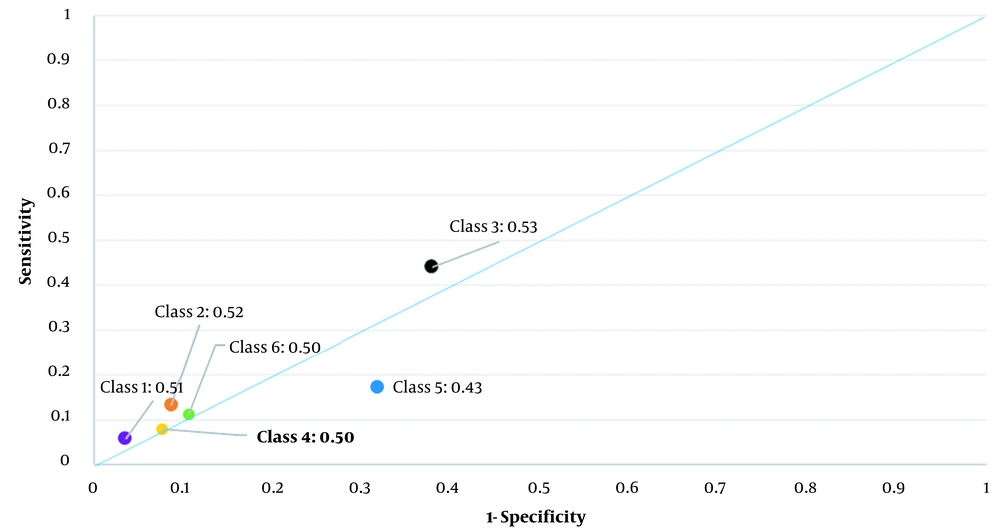
In Figure 2, the AUC of all the clinical symptom patterns was plotted. The AUC of the symptom patterns was poor, being 0.43 for class 5, comprising patients without any symptoms, and 0.53 for class 3, comprising patients with fever, chills, and cough. Table 4 shows the Se, Sp, and the AUC of all the extracted clinical symptom patterns by LCA across the age groups. The Se, Sp, and AUC of each age group were different due to the different relative frequency of confirmed COVID-19 in each age group (Table 4). For example, the age group < 20 years had the lowest relative frequency of confirmed COVID-19 (5.1%), and class 3 had the highest Se and AUC. While the 50 - 59 years age group had the highest relative frequency of confirmed COVID-19 (42.0%), class 3 had the highest Se and AUC. Almost every class showed similar diagnostic performance (Se, Sp, and AUC) across the age groups. For example, Se, Sp, and AU performances in class 1 were as follows: An Se value of 4.8% in the < 20 years age group and 7.4% in the 40 - 50 years age group; an Sp value of 95.4% in the 20 - 30 years age group and 97.5% in ≥ 80 years age group; and an AUC value of 50.6% in the 20 - 30 years age group and 51.5% in the 40 - 50 years age group.
The area under the receiver operating characteristic curve for clinical symptom patterns extracted by latent class analysis among hospitalized patients with suspected COVID-19 and 1- specificity on the X-axis and sensitivity on the Y-axis; Class 1: Patients with fever, chills, cough, shortness of breath, sore throat, and arthralgia; Class 2: Patients with fever, chills, cough, and shortness of breath; Class 3: Patients with fever, chills, and cough; Class 4: Patients with fever and chills; Class 5: Patients without any symptoms; Class 6: Patients with myalgia
| Age Groups | |||||
|---|---|---|---|---|---|
| < 20 | 20 - 39 | 40 - 59 | 60 - 79 | ≥ 80 | |
| Patients a | 702 (5.1) | 3,107 (22.6) | 4,540 (33.1) | 3,883 (28.3) | 1,492 (10.9) |
| RT-PCR+ a | 145 (20.7) | 1,114 (35.9) | 1,906 (42.0) | 1,296 (33.4) | 375 (25.1) |
| Syndromic Patterns | |||||
| Class 1 b | |||||
| Se | 4.8% | 5.2% | 7.4% | 4.9% | 4.0% |
| Sp | 97.3% | 95.4% | 95.7% | 97.1% | 97.5% |
| AUC | 51.1% | 50.6% | 51.5% | 51.0% | 50.8% |
| Class 2 c | |||||
| Se | 9.7% | 12.5% | 14.2% | 14.3% | 9.3% |
| Sp | 95.5% | 92.4% | 91.6% | 89.8% | 90.6% |
| AUC | 52.5% | 52.4% | 52.8% | 52.1% | 50.0% |
| Class 3 d | |||||
| Se | 42.7% | 47.1% | 45.4% | 41.6% | 37.6% |
| Sp | 64.5% | 57.2% | 57.6% | 66.6% | 69.8% |
| AUC | 53.6% | 52.2% | 51.5% | 54.1% | 53.7% |
| Class 4 e | |||||
| Se | 19.3% | 7.6% | 7.6% | 7.7% | 8.8% |
| Sp | 81.7% | 93.4% | 93.5% | 92.8% | 92.1% |
| AUC | 50.5% | 50.5% | 50.5% | 50.3% | 50.5% |
| Class 5 f | |||||
| Se | 16.5% | 14.6% | 14.1% | 21.1% | 30.4% |
| Sp | 68.4% | 71.5% | 72.9% | 64.3% | 60.3% |
| AUC | 42.4% | 43.1% | 43.5% | 42.7% | 45.4% |
| Class 6 g | |||||
| Se | 6.9% | 12.9% | 11.4% | 10.3% | 9.9% |
| Sp | 92.6% | 89.5% | 88.6% | 89.3% | 89.4% |
| AUC | 49.8% | 51.2% | 50.0% | 49.8% | 49.5% |
The Sensitivity, Specificity, and Area Under the Receiver Operating Characteristic Curve of COVID-19 Syndromic Patterns Across the Age Groups
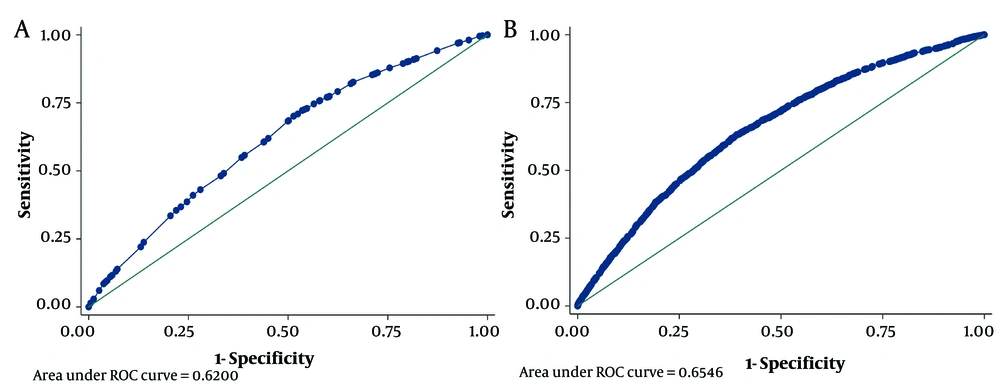
Table 5 shows a predictive COVID-19 model for the classes of clinical symptoms (model 1) and each symptom among the patients (model 2) by multiple logistic regression. For example, in model 1, class 1 comprising patients with fever, chills, cough, shortness of breath, sore throat, and arthralgia had an odds ratio of 2.87 (1.39, 3.43) relative to class 5 (patients without any symptoms). Figure 3 presents the accuracy of each model (models 1 and 2) for COVID-19 prediction.
| Variables | OR (95% CI) |
|---|---|
| Model 1 | |
| Classes (ref: Class 5) | |
| Class 1 | 2.87 (2.39, 3.43) a |
| Class 2 | 2.69 (2.36, 3.07) a |
| Class 3 | 2.02 (1.84, 2.22) a |
| Class 4 | 1.99 (1.72, 2.31) a |
| Class 6 | 1.83 (1.6, 2.09) a |
| Gender (ref: female) | 0.98 (0.91, 1.05) |
| Age Groups (ref: < 20) | |
| 20 - 39 | 2.08 (1.7, 2.53) a |
| 40 - 59 | 2.65 (2.18, 3.22) a |
| 60 - 79 | 1.94 (1.59, 2.36) a |
| ≥ 80 | 1.36 (1.09, 1.69) b |
| Intercept | 0.15 (0.12, 0.19) a |
| Model 2 | |
| Symptoms | |
| Fever and chills | 1.78 (1.66, 1.92) a |
| Cough | 1.52 (1.41, 1.64) a |
| Shortness of breath | 1.41 (1.31, 1.52) a |
| Fatigue | 1.1 (0.99, 1.22) |
| Myalgia | 1.16 (1.05, 1.28) b |
| Irritability or confusion | 0.88 (0.76, 1.02) |
| Sore throat | 0.97 (0.88, 1.07) |
| Diarrhea | 1.42 (1.19, 1.69) a |
| Nausea or vomiting | 1.16 (1.01, 1.32) c |
| Headache | 1.33 (1.19, 1.5) a |
| Arthralgia | 0.79 (0.67, 0.92) b |
| Other symptoms | 1.21 (1.1, 1.34) a |
| Gender (ref: female) | 0.96 (0.89, 1.03) |
| Age groups (ref: < 20) | |
| 20 - 39 | 2.15 (1.75, 2.63) a |
| 40 - 59 | 2.68 (2.2, 3.27) a |
| 60 - 79 | 1.94 (1.59, 2.37) a |
| ≥ 80 | 1.36 (1.09, 1.7) b |
| Intercept | 0.12 (0.1, 0.15) a |
A Predictive Model for COVID-19 for Classes of Clinical Symptoms (Model 1) and Each Symptom Among Patients (Model 2)
5. Discussion
The results of this study demonstrated the diagnostic value of the COVID-19 clinical symptoms and their patterns to predict confirmed COVID-19 cases in northern Iran. We presented and compared the Se and Sp of each recorded clinical symptom in the surveillance system of COVID-19 (Iran CDC) in northern Iran and categorized hospitalized patients with suspected COVID-19 according to significant clinical symptoms in six classes by LCA.
The prevalence of the COVID-19 clinical symptoms was almost similar among the hospitalized patients in this study and other studies in Iran (18-20). However, the prevalence of fever, cough, myalgia, shortness of breath, and sore throat was less in this study than in studies in other countries (21-24).
This study showed that the clinical symptoms may not help predict confirmed COVID-19. In other words, the suggested clinical symptoms of surveillance systems were not informative for diagnosis. Also, the extracted patterns of significant clinical symptoms were not informative for diagnosis. The reason is that the AUC of the classes was less than 0.53. In addition, the predictive models of all the clinical symptoms and their patterns showed poor results (AUCs less than 0.65).
Consistent with previous studies in China (7, 25, 26), fever and cough were the most common symptoms in patients infected with COVID-19. Concerning the pandemic situation of COVID-19, case definitions with higher Se are preferred. However, our study did not show any pattern of clinical symptoms (classes) of a highly sensitive case definition. Indeed, it is desirable to use case definitions with good diagnostic performance in all age groups in pandemic situations. In contrast, the current study showed that these clinical symptoms had a high Sp.
On the other hand, the population in the current study included suspicious hospitalized patients of COVID-19 (cases with clinical and without sub-clinical manifestations) referred from the first level of the surveillance system (primary health care centers or outpatient centers) to the hospital. We expected a high homogeneity for participation and a fewer number of classes in this study by LCA. The analyses showed the high heterogeneity of the patients according to the clinical symptoms. Thus, these findings can have at least four justifications: (1) Referral of unrelated cases (noncompliance with the WHO-suspected-COVID-19 case definition criteria) from the first-level of the surveillance system (primary health care centers or outpatient centers) to the hospital; (2) the poor performance of hospitals for detection of positive cases; (3) incomplete or inaccurate recording of clinical symptoms or failure to record them in patients; (4) the inadequacy of suggested clinical symptoms for case definition in the surveillance system of Iran.
In the current study, class 3 (patients with clinical symptom patterns of fever, chills, and cough) had the best performance (AUC) and the highest Se compared to the other classes (case definitions). On the other hand, although the prevalence (relative frequency) of confirmed COVID-19 was various in the age groups, almost every class showed similar diagnostic performance across the age groups. This result may be justified by the findings noted above. The reason is that patients with age ≥ 60 years showed obvious clinical manifestations in previous studies (27, 28), although inconsistent with our findings (Appendix 2: The frequency and percentage of clinical symptom manifestations across the age groups with confirmed COVID-19). This is important because clinical features of the disease vary across age groups, and young age groups are asymptomatic (29). Thus, they may be more active in transmitting COVID-19 to others in the population.
Among the reasons for this heterogeneity in the hospitalized patients were the untrained medical staff, not following a specific guideline, and not allocating specific hospitals for admission and care of patients with COVID-19 (30). However, these problems were resolved over time, and a number of hospitals in the province were specifically designated for admission and care of COVID-19 patients. Loss of taste and smell in the following months as an essential symptom of COVID-19 infection verified this finding. Also, we suggest analyzing the clinical manifestation of the disease over time regarding this finding.
Using parallel tests may increase the diagnostic performance. However, we did not observe this enhance performance regarding COVID-19 diagnosis in the current study because both predictive models had AUCs less than 0.65.
5.1. Limitations
This study had several limitations. First, the data in the study was collected through self-report by the patient or their caregiver and not by a thorough examination of the patient by a physician. Second, the available data on the clinical symptoms was based on the initial WHO recommendation in previous studies for similar diseases such as influenza. Unfortunately, there were no signs of symptoms like anosmia and hypogeusia as suggested clinical symptoms in the checklist of the surveillance system of CDC in IRAN, when this study was conducted. The reason is that recent studies (31, 32) have shown anosmia and hypogeusia as common symptoms in confirmed COVID-19 cases. Third, the data in this study only included symptoms recorded in the medical records of the surveillance system of CDC in IRAN. We suggest the diagnostic evaluation of clinical signs and laboratory findings. Fourth, there were problems with the accuracy of reports, which may be due to the high volume of work during the pandemic crisis and emergencies, lack of skilled and trained workforce, or lack of commitment of some personnel to record data in the surveillance system of the Center for Disease Management. It is suggested to evaluate the degree of data quality through bias analysis and measure the invariance of LCA across negative and positive COVID-19 using RT-PCR and over time. Fifth, although LCA is an alternative method for determining test characteristics, the diagnostic measures may not be generalizable to the total population because the study population was not a spectrum of healthy and diseased individuals. Sixth, although real-time RT-PCR is considered a primary method to detect the causative agent of COVID-19, SARS-CoV-2, its Se and Sp are not 100% (10). Thus, the false negative may be expected due to various limitations noted above. Thus, the diagnostic evaluation of clinical symptoms by chest CT and RT-PCR is suggested. Previous studies (33, 34) showed a Se of 98% for chest CT. Finally, the subjects of this study were hospitalized patients, and thus the generalizability of the findings to outpatients is doubtful and has to be examined.
5.2. Conclusions
The findings of this study showed that the extracted patterns of the COVID-19 clinical symptoms in hospitalized patients with suspected COVID-19 (including definitive patients) in northern Iran had a low diagnostic value for case definitive diagnosis. Also, the predictive models showed that the clinical symptoms may not help predict confirmed COVID-19. The suggested clinical symptoms in the surveillance form are inadequate, and we suggest excluding non-informative clinical symptoms and replacing them with alternative symptoms such as anosmia and hypogeusia in the surveillance form. These symptoms could not explain the class separation and homogeneity of the LCA models in the present data. Thus, most of these symptoms are not informative for diagnostic purposes, possibly due to inaccurate or incomplete recording of clinical symptoms or failure to record them in patients. Also, this study’s results showed that the designed surveillance system could not collect good information about COVID-19 in the initial months in Iran. The results can help revise and improve COVID-19 surveillance in Iran.