1. Background
Given the prevalence of acute cholecystitis globally and the high mortality of its patients, it is essential to study this disease and its appropriate treatment (1, 2). Acute cholecystitis is one of the most common causes of hospitalization. If acute cholecystitis is not diagnosed in time or treated improperly, the patient may develop a more serious clinical condition such as cholangitis, sepsis, and organ dysfunction. Thus, early diagnosis and appropriate treatment of the disease are critical (3). The disease is an inflammation of the bile ducts secondary to obstruction. In 80% of patients, this obstruction is caused by gallstones (4). In the United States, 20.5 million people, including 6.3 million men and 14.2 million women, have gallstones, and one million people are diagnosed as new patients each year (5). Biliary obstruction increases the pressure inside the ducts and leads to the spread and proliferation of microbes (6). Bile is usually completely sterile, but the presence of bacteria in the bile ducts is a common finding in acute cholecystitis. Even in many patients with no evidence of gallbladder infection at the time of surgery, histological changes in the gallbladder wall are seen based on infectious processes (7-9). Bactibilia is essential in postoperative septic complications such as gram-negative septicemia and other postoperative infections (10).
Many patients with laparoscopic cholecystectomy use many antibiotics before and after surgery without basic information about the causes of acute cholecystitis, antibiotic resistance, and susceptibility. Improper use of antibiotics is one of the causes of microbial resistance, leading to increased mortality and morbidity. It is possible to reduce the inappropriate use of antibiotics and microbial resistance by correctly recognizing the common agents of bile samples in patients with acute cholecystitis and their antibiograms (1).
2. Objectives
Antimicrobial resistance is one of the most critical issues in the global health community. Over the past decade, the prevalence of multidrug-resistant bacteria has increased dramatically with the problem of nosocomial infections (11). Since few studies have been conducted in this field in Iran, this study was performed to identify microbes responsible for acute cholecystitis and their antibiogram.
3. Methods
This cross-sectional study was performed at the Imam Hossein Educational Hospital, Tehran, Iran, between 2019 - 2020. This study included 97 patients with acute cholecystitis referred to the Imam Hossein Hospital. Inclusion criteria were patients of all age groups and genders who were clinically and paraclinically diagnosed with acute cholecystitis. Exclusion criteria included patients admitted with a diagnosis of acute cholecystitis but discharged in good general condition without the need for cholecystectomy.
A surgeon made the diagnosis of acute cholecystitis using history and ultrasound. All our patients received intravenous antimicrobial prophylaxis with 1gram ceftriaxone and 250 milligram metronidazole preoperatively. During surgery, a surgeon aspirated 5 ml of the bile samples with a sterile syringe from the gallbladder immediately after cholecystectomy and placed it in a sterile transport medium. The samples were sent immediately to the microbiology laboratory and then cultured in two media, aerobic and anaerobic. After the bacteria were isolated, we performed antibiotic sensitivity tests with a colony in Mueller-Hinton agar medium (manufactured by Merck). The applied antibiotic disks were ampicillin-sulbactam, amikacin, gentamicin, cefotaxime, cefepime, ceftazidime, vancomycin, ceftriaxone, imipene, meropenem, piperacilin-tazobactam, ciprofloxacin, and co-amoxiclave. The researcher recorded each patient’s information including name and surname, age, sex, history of diabetes, history of liver disease and gallbladder and bile ducts, history of previous antibiotics by the patient, bile slug and stone in ultrasonography, culture, and antibiogram of bile samples. The results were analyzed using SPSS 16 software.
4. Results
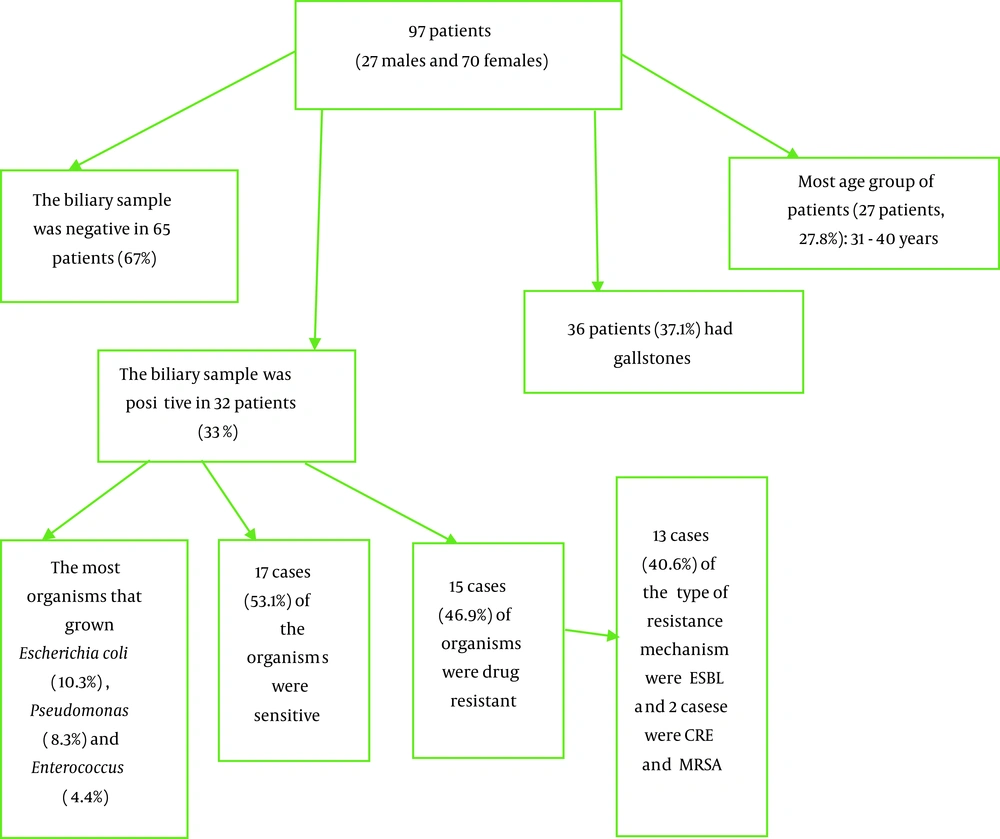
In this study, 97 patients (27 male and 70 female) were studied. Of the patients, nine (9.3%) had diabetes, four (4.1%) had a history of previous cholecystitis, and four (4.1%) had a history of antibiotic use in the last ten days. Most of the patients (27 patients, 27.8%) were aged 31 - 40 years. Thirty-six (37.1%) of the patients had gallstones and increased thickness of the gallbladder wall on abdominal ultrasound (Table 1) (Figure 1).
| Variables | No. (%) |
|---|---|
| Age | |
| 20 - 30 | 20 (20.6) |
| 31 - 40 | 27 (27.8) |
| 41 - 50 | 18 (18.6) |
| 51 - 60 | 15 (15.5) |
| 61 - 70 | 12 (12.4) |
| 71 - 80 | 4 (4.1) |
| 81 - 90 | 1 (1) |
| Total | 97 (100.0) |
| Sex | |
| Male | 27 (27.83) |
| Female | 70 (72.16) |
| Total | 97 (100) |
| Diabetes mellitus | |
| Yes | 9 (9.3) |
| No | 88 (90.7) |
| Total | 97 (100) |
| History of cholecystitis | |
| Yes | 4 (4.1) |
| No | 93 (95.9) |
| Total | 97 (100) |
| History of antibiotics use | |
| Yes | 4 (4.1) |
| No | 93 (95.9) |
| Total | 97 (100) |
| Galbllader stone | |
| Yes | 36 (37.1) |
| No | 61 (62.9) |
| Total | 97 (100) |
The result of the biliary sample of patients with the sterilized method was negative in 65 (67%) of the patients, and organisms grown were primarily Escherichia coli (10 people, 10.3%), Pseudomonas (eight people, 8.3%), and Enterococcus (four people, 4.4%). In 32 cases with a positive culture, 17 (53.1%) cases were sensitive and without drug resistance. Also, the type of resistance mechanism reported extended-spectrum beta-lactamase (ESBL) in 13 (40.6%) cases and carbapenem-resistant enterobacteriaceae (CRE) and methicillin-resistant Staphylococcus aureus (MRSA) in two cases (Table 2).
| Variables | No. (%) |
|---|---|
| Culture | |
| Negative | 65 (67) |
| Escherichia coli | 10 (10.3) |
| Enterococcus | 4 (4.1) |
| Pseudomonas | 8 (8.2) |
| Enterobacter | 3 (3.1) |
| Klebsiella | 3 (3.1) |
| Etc (Providencia, Streptococcus viridans, Citrobater frendii, Staphylococcus aureus) | 4 (4.1) |
| Total | 97 (100) |
| Resistance mechanism | |
| Sensitive | 17 (53.1) |
| ESBL | 13 (40.6) |
| CRE | 1 (3.12) |
| MRSA | 1 (3.12) |
| Total | 32 (100) |
The distribution of the patients based on the culture result (positive and negative) and the studied variables is shown in Table 3. By using the chi-square test or Fisher's exact test, it was found that only the difference between the age group and the positive or negative culture was statistically significant (P < 0.032).
| Variables | Negative Culture (n = 65) | Positive Culture (n = 32) | P Value |
|---|---|---|---|
| Age | 0.032 | ||
| 20 - 30 | 14 (21.5) | 6 (18.8) | |
| 31 - 40 | 22 (33.8) | 5 (15.6) | |
| 41 - 50 | 11 (16.9) | 7 (21.9) | |
| 51 - 60 | 11 (16.9) | 4 (12.5) | |
| 61 - 70 | 7 (10.8) | 5 (15.6) | |
| 71 - 80 | 0 (0) | 4 (12.5) | |
| 81 - 90 | 0 (0) | 1 (3.1) | |
| Sex | |||
| Male | 14 (21.5) | 9 (28.1) | 0.061 |
| Female | 51 (78.5) | 23 (71.9) | |
| Diabetes mellitus | 0.472 | ||
| Yes | 5 (7.7) | 4 (12.5) | |
| No | 60 (92.3) | 28 (87.5) | |
| Antibiotic use | 0.597 | ||
| Yes | 2 (3.1) | 2 (6.3) | |
| No | 63 (96.9) | 30 (93.8) | |
| History of cholecystitis | 0.299 | ||
| Yes | 4 (6.2) | 0 (0) | |
| No | 61 (93.8) | 32 (100.0) | |
| Gallbladder stone | 1.000 | ||
| Yes | 24 (36.9) | 12 (37.5) | |
| No | 41 (63.1) | 20 (62.5) |
a Values are expressed as No. (%).
The distribution of positively cultured patients based on the type of microorganism and the studied variables is shown in Table 4. By using the Chi-square test or Fisher's exact test, it was found that the difference was significant between different organisms with the sex of patients (P < 0.032) and having gallstones (P < 0.011).
| Variables | Escherichia coli | Enterococcus | Pseudomonas | Enterobacter | Klebsiella | etc. | P Value |
|---|---|---|---|---|---|---|---|
| Age | 0.212 | ||||||
| 20 - 30 | 0 (0) | 0 (0) | 3 (37.5) | 1(33.3) | 1(33.3) | 0 (25) | |
| 31 - 40 | 1 (10) | 1 (25) | 1 (12.5) | 2 (66.7) | 0 (0) | 0 (0) | |
| 41 - 50 | 1 (10) | 1 (25) | 2 (25) | 0 (0) | 1 (33.3) | 1 (50) | |
| 51 - 60 | 1 (10) | 0 (0) | 1 (12.5) | 0 (0) | 1 (33.3) | 1 (50) | |
| 61 - 70 | 3 (30.0) | 1 (25) | 2 (25) | 0 (0) | 0 (0) | 0 (0) | |
| 71 - 80 | 4 (40) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 81 - 90 | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Sex | 0.032 | ||||||
| Male | 5 (50) | 3 (75) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | |
| Female | 5 (50) | 1 (25) | 8 (100) | 3 (100) | 3 (100) | 3 (75) | |
| Diabetes mellitus | 0.441 | ||||||
| Yes | 3 (30) | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) | |
| No | 7 (70) | 4 (100) | 7 (87.5) | 3 (100) | 3 (100) | 4 (100) | |
| Antibiotic use | 0.454 | ||||||
| Yes | 2 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| No | 8 (80) | 4 (100) | 8 (100) | 3 (100) | 3 (100) | 4 (100) | |
| History of cholecystitis | 0.462 | ||||||
| Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| No | 10 (100) | 4 (100) | 8 (100) | 3 (100) | 3 (100) | 4 (100) | |
| Gallbladder stone | 0.011 | ||||||
| Yes | 6 (40) | 4 (100) | 1 (12.5) | 0 (0) | 0 (0) | 1 (25) | |
| No | 4 (0) | 0 (0) | 7 (87.5) | 3 (100) | 3 (100) | 3 (75) |
a Values are expressed as No. (%).
5. Discussion
In this study, out of 97 patients, 27.83% were male and 72.16% female, 9.3% had diabetes, 4.1% had a history of previous cholecystitis, and 4.1% had a history of antibiotics during the last ten days. Most of the patients (27 patients, 27.8%) were in the age group of 31-40 years. Thirty-six (37.1%) of the patients had gallstones and increased thickness of the gallbladder wall on abdominal ultrasound. Among the variables, only the difference between the age category and positive or negative culture was statistically significant (P < 0.032). In a study conducted by Moazeni-Bistgani and Imani in 2013 at the Shahrekord University of Medical Sciences, the population included 88 (66.7%) women and 44 (33.3%) men, with the mean age of 55.6 ± 14.3 years (range; 18 and 63 years old) (12). In Parekh et al.’s study the maximum age of patients was between 41 - 50 years. Isolation of bile from the bile was most common in the age group of 19 - 30 years, and females were predominant (13). Also, in another study, Dr. Rubén Cueto-Ramos et al. examined 183 patients in North American Mexico in 2016, of whom 151 (82.5%) were women and 32 (17.5%) men, with an average age of 35 years. The most common underlying diseases were hypertension (13.1%), diabetes mellitus (9.2%), bile pancreatitis (4.9%), and gallstone (7.1%) (14). Shoorashetty and Pushpalatha conducted a study on 50 patients in India in 2012, of whom 31 (62%) were female and 19 (38%) male, with the average age being 15 - 45 years. Gallstones were diagnosed in 28 patients, gallstones in 12 patients, neoplasms in six patients, and bile duct stenosis in four patients (15).
In our study, the result of bile sample culture was negative in 67% of the patients. In other positive samples, organisms grown were mainly E. coli, Pseudomonas, and Enterococcus. In cases where the culture was positive, 53.1% of the organisms were susceptible and without antibiotic resistance. In cases with drug resistance, the highest resistance mechanism was ESBL type and several cases of them were CRE and MRSA types. The relationship between the type of organisms and sex of patients (P < 0.032) and gallstones (P < 0.011) was significant. While in a study conducted by Moazeni-Bistgani and Imani in 2013 at the Shahrekord University of Medical Sciences in Iran, 37.87% of the total sample size had a positive culture. The most common microorganisms were E. coli (26%), followed by Enterobacteriaceae (18%) and Salmonella typhi (14%) (12). Another study by Parekh et al. in 2015 in India found that 50% of patients had a positive culture. The most common microorganisms were E. coli (63.16%), Pseudomonas (3.85%), Klebsiella (2.56%), and coagulase-negative staphylococci and Streptococcus viridans (1.28%). In this study, the most effective antibiotics were 3rd and 4th generation cephalosporins, levofloxacin, and piperacillin-tazobactam. Also, the highest antibiotic resistance was to aminoglycosides and 2nd generation cephalosporins (13). In the study carried out by Dr. Rubén Cueto-Ramos et al. in 2016, 68.3% had negative cultures with an enterobacteriacea group, 43.0%, and enterococci were positive for 27.58%. There was a significant relationship in the severity of the disease symptoms between the two groups of positive and negative cultures. This study finally advised prescribing metronidazole and fluoroquinolones in suspected patients with biliary infections and patients with risk (14). In another study conducted by Shoorashetty and Pushpalatha in India in 2012, bacteria were observed in 52% of bile samples. Also, 10% of the samples were polymicrobial and included E. coli, Klebsiella pneumonia, and Enterococcus faecalis. None of the anaerobes were isolated. The resistance mechanism has been reported to be ESBL in 47% and Amp C in 31.5% of cases (15). Among the limitations of this study was the lack of laboratory facilities in the center for molecular evaluation of various antibiotic resistances.
5.1. Conclusions
According to what was mentioned in the discussion section, cholecystitis was more common in females than in males and in the age group of 20 - 40 years, although insignificant. There are not many studies on the underlying diseases of diabetes, hypertension, hyperlipidemia, pancreatic disease, and type of nutrition in patients with cholecystitis, and there was no significant relationship between the reviewed studies. Microbiologically, according to studies, 30 - 50% of cholecystitis cultures of bile samples were positive. The most common organisms growing in these cultures include E. coli, Pseudomonas, Klebsiella pneumonia, Enterococcus, and Salmonella species. Also, the most common mechanism of antibiotic resistance is ESBL type, followed by CRE and AmpC types.
The study's objectives were to collect data to identify the percentage of bacterial infections of acute cholecystitis in the center and detect the most common causative bacteria and their pattern of antibiotic resistance. Further research needs to be done on the prevalence of microbes in the center in cholecystitis and other diseases and the selection of appropriate antibiotic regimens and mechanisms of antibiotic resistance and to prevent the over-administration of antibiotics.