1. Background
Respiratory and burn infections caused by Pseudomonas aeruginosa are a major source of concern globally (1, 2). According to numerous studies, the mortality rate of ventilator-associated pneumonia (VAP) with P. aeruginosa is higher than 13.5% (3). Moreover, burn victims can immediately become infected with a systemic infection with a 38 to 70% mortality rate (4). It is noted that the treatment of P. aeruginosa infections with the conventional use of antibiotics has become a significant challenge due to the ability of this bacterium to resist (5-7). The intrinsic and acquired resistance in this bacterium consists of producing antibiotic-inactivating enzymes, expression of efflux pumps, low permeability in the outer membrane, and mutations and horizontal transfers of resistance genes. The formation of biofilm causes a diffusion barrier to restrict antibiotic entrance to bacterial cells. Furthermore, multidrug-tolerant persister cells are subject to prolonged and recurrent infections in cystic fibrosis patients (8). Therefore, producing an efficacious vaccine or another alternative immunotherapy to combat the spread of P. aeruginosa infection is necessarily required.
So far, numerous vaccine candidates, including pili, flagella, alginate, outer membrane proteins (OMPs), etc., have been examined for P. aeruginosa. Since P. aeruginosa uses various pathways to produce infection and escape from immune system particles, no report authorizes a vaccine (9). OprF can bind peptidoglycan to the outer membrane, and OprI can play an essential role in susceptibility to antimicrobial peptides (10). These fragments have been selected to induce various immune effectors, including opsonophagocytic killing antibodies. Moreover, the PopB of the type III secretion system has been chosen to incite high interleukin 17 (IL-17) production, increasing the recruitment of neutrophils to the site of inflammation and protecting the animal model from lethal P. aeruginosa. Since the interaction between secretory proteins or exposed surfaces with host cells is critical in pathogenicity, bioinformatics approaches and tools can apply to obtain new insights into these interactions and their performances in vaccine design investigations.
Due to immune modulation effects, recruitment of dendritic cells (DCs), migration to lymph node regions, and low toxicity profiles, the granulocyte-macrophage colony-stimulating factor (GM-CSF) displays an attractive vaccine adjuvant (11).
2. Objectives
Algorithm development to create new vaccines is one of the principal bioinformatics objectives. Recognizing reliable epitopes through bioinformatics analysis decreases the costs of laboratory testing. In the present study, bioinformatics tools were implemented to construct a proper vaccine using OprF, OprI, and PopB proteins of P. aeruginosa, accompanied by GM-CSF as an adjuvant. In this regard, according to these protein structures, the main physicochemical and immunological epitopes were predicted and evaluated, resulting in a new structure as a vaccine candidate against P. aeruginosa.
3. Methods
3.1. Protein Sequence Retrieval
Antigens acquired for this study are as follows: OprF (accession number: P13794), OprI (accession number: P11221), and PopB (accession number: Q9I324) as primary sequences and GM-CSF (accession number: A0A0B4U6M2) as an adjuvant. The protein sequences were obtained from UniProtKB (https://www.uniprot.org) and then stored as a FASTA format for additional analysis.
3.2. B-Cell (Linear) Epitope Prediction
B-lymphocytes play a crucial role in recognizing soluble antigens by producing antibodies. Neutralization and phagocytosis with or without complement can occur by these components. Two forms of B-cell epitopes were identified, consisting of linear B-cell epitopes (containing sequential residues) and conformational (discontinuous) B-cell epitopes (including patches of solvent-accessible atoms). The accurate identification of linear B-cell epitopes was achieved by utilizing physicochemical properties of amino acids via BcePred software (http://ailab.ist.psu.edu/bcpred/predict.html) (12) and ABCpred server (http://crdd.osdd.net/raghava//abcpred/) with an artificial neural network (cutoff score > 0.7) (13).
3.3. Assessment of Antigenicity and Allergenicity
Since antigenicity evaluation is the main stage of vaccine design, VaxiJen version 2.0, AllerTOP version 2, and AlgPred online server prediction were used to ensure that the vaccine did not evoke any allergic response (14-16).
3.4. Solubility and Physicochemical Parameters Prediction
To evaluate various physicochemical characteristics of the vaccine sequence, Protein-Sol (http://protein-sol.manchester.ac.uk) (17) and ProtParam (http://web.expasy.org/protparam/) (18) were used. The population average for Protein-Sol was 0.45 (as a default), and Protein-Sol computed various chemical and physical parameters, including the molecular weight, grand average of hydropathicity (GRAVY), and so on.
3.5. Protein Secondary Structure Prediction
To estimate the secondary structural contents, the GOR V server (http://cib.cf.ocha.ac.jp/bitool/GOR/) based on the Garnier, Osguthorpe, and Robson method was used (19).
3.6. Vaccine 3D Structure Prediction
Predicting the 3D structure of the protein was developed using the Iterative Threading ASSEmbly Refinement (I-TASSER) server, which experimentally identifies the query protein from the template structure of the Protein Data Bank (PDB) via incorporating the LOcal MEta-Threading Server (LOMETS) (20). To visualize the 3D structure, we applied the PyMol software package (21).
3.7. Protein Structure Quality and Validation
The refinement process for the selected protein model was examined in the GalaxyRefine web server (http://galaxy.seoklab.org/). The primary proteins rebuilt side chains via locating the highest-probability rotamers, produced side-chain repacking, and relaxed the refinement construct via 2 methods: moderate relaxation or an aggressive one (22). The ultimate model was validated by the following computational tools. The ProSA-web server (https://prosa.services.came.sbg.ac.at/prosa.php) was used to recognize errors in the 3D models. Overall model quality, outside a range, is essential for primary proteins presenting inaccurate structures (23). The identification of disfavored protein side-chain rotamers, all-atom contact assessment with specific hydrogens (consisting clash score), Ramachandran plot, and MolProbity score were calculated via the MolProbity structure-validation web service.
3.8. B-Cell (Discontinuous or Conformational) Epitope Prediction
The ElliPro web-accessible server (http://tools.iedb.org/ellipro/) was implemented as a web-accessible application to predict 3D structures via the determination of the protrusion index (PI; residues with highest scores chosen) and clustered based on their PI values and the distance R of each (24).
3.9. Major Histocompatibility Complex Class I Binding (Cytotoxic T Lymphocyte) Epitopes Prediction
The prediction of cytotoxic T lymphocyte (CTL) epitopes in OprF, OprI, and PopB proteins of P. aeruginosa was performed using the NetCTL 1.2 server (http://www.cbs.dtu.dk/services/NetCTL/), which has a crucial role in bacterial clearance in cystic fibrosis. This server is connected with antigen processing [transporter associated with antigen processing (TAP)] transport or proteasomal cleavage mechanisms to evaluate the prediction of antigen processing and presentation via the major histocompatibility complex (MHC) class I pathway. Weight on cleavage in this method was estimated at 0.15, epitope identification value at 0.72, and TAP transport at 0.05 (25).
3.10. MHC Class II Binding (Helper T Lymphocyte) Epitopes Prediction
The 15-mer helper T lymphocyte (HTL) of structural OMPs and PopB was recognized by applying the IEDB web server (http://tools.iedb.org/mhcii/), a popular MHC class II binding prediction tool. The sum of 7 reference sets of human leukocyte antigen (HLA) alleles of the related server, including HLA-DRB1 (*03: 01, *07: 01, and *15: 01), HLA-DRB3 (*01: 01 and *02: 02), HLA-DRB4*01: 01, and HLA-DRB5*01: 01, was preferred for MHC II binding epitope prediction. The stabilized matrix method (SMM) was used to evaluate the half-maximal inhibitory concentration (IC50) value of the selected peptide connecting to MHC-II alleles, and the IC50 value less than 500 nM was considered a good affinity (26).
3.11. Interferon Gamma Prediction
The interferon-gamma (IFN-γ) cytokine plays a significant role in combating chronic P. aeruginosa infection by adjusting immune response approaching an overall T helper (Th) 1 pattern. The production of IFN-γ induced activation of macrophages, DCs, and natural killer (NK) cells. The IFN-γ epitope server (webs.iiitd.edu.in/raghava/ifnepitope/application.php) was applied to distinguish the low scores of HTL epitopes. Positive results were predicted based on the hybrid strength of support vector machine (SVM) and motif-based techniques. Therefore, the ultimate HTL epitopes were chosen based on MHC class II binding and IFN-γ induction, both of which promote the stimulation of Th cells (27).
3.12. Immune Simulation
To predict the final vaccine-elicited immune responses in natural conditions, computational modeling on the human immune system was accomplished via the C-ImmSim online server (https://kraken.iac.rm.cnr.it/C-IMMSIM/). Due to the Celada-Seiden model available in this server, humoral and cellular immunity of mammalian host defense can be represented upon a vaccine design. Three injections during 2-week intervals, including 1000 vaccine proteins, were administered through the simulation default parameters (with time step set at 1, 84, and 170 with a sum of 1050 simulation steps). Furthermore, the production of antibodies and cytokines was determined upon the injection of the chimeric vaccine (28).
3.13. Interaction Analysis of Chimeric Vaccine with TLR4
A molecular docking study was implemented using the ClusPro server (https://cluspro.bu.edu/login.php?redir/queue.php). Docking was performed with TLR4 (PDB ID code: 4G8A), which acts as a receptor to recognize antigenic molecules. This server is a useful computational tool that investigated the models based on desolvation energy and electrostatic interactions. The refined complexes were presented by ClusPro based on numerous agents, including atomic contact energy, hydrophobic-favored, electrostatic-favored, and VDW plus ELEC (29).
3.14. Molecular Dynamics of Receptor-Vaccine Complex Simulation
The molecular dynamics (MD) simulation study was administered for the subunit vaccine structure manifested the best molecular docking investigation outcomes. The iMODS web server (http://imods.Chaconlab.org/) was used to determine and assess protein flexibility (30).
3.15. Codon Optimization and In Silico Vaccine Expression
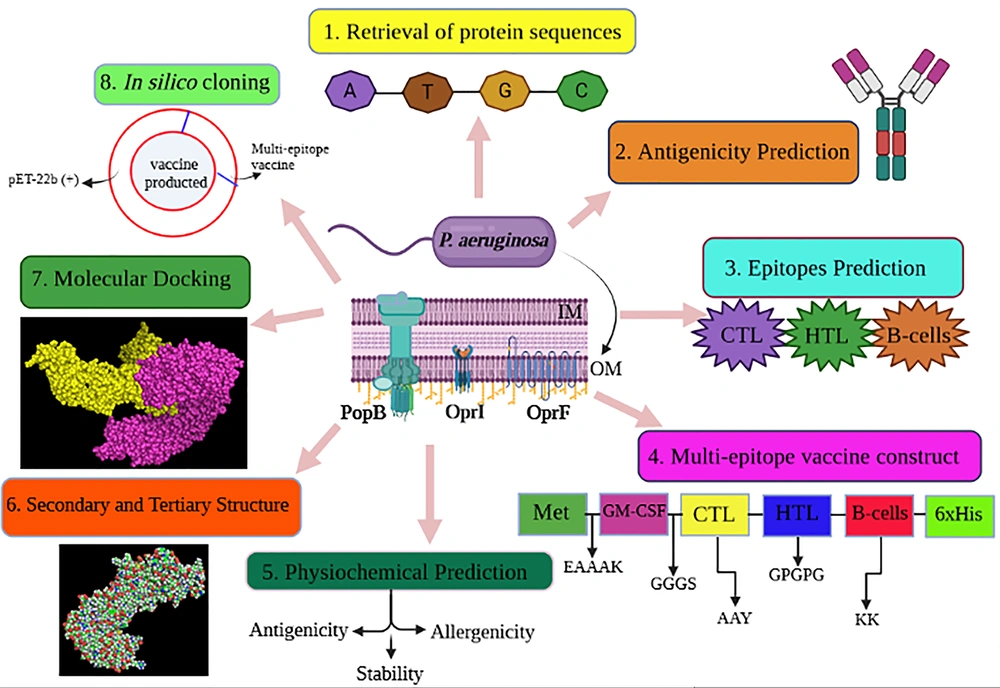
The optimization of the codon procedure was applied to express the recombinant protein using the Codon Adaptation Tool (JCat) server (http://www.jcat.de/). The codon adaptation index (CAI) values with the best value of 1.0 and guanine-cytosine (GC) contents ranging from 30 - 70% were used to manage protein expression levels (31). It is noted that the result beyond this scope does not have good efficiencies on translation and transcription steps. The chimeric vaccine construct was optimized based on the bacterial expression host and Escherichia coli codon usage by Gene Designer-v1 software. After codon optimization, HindIII and KasI restriction sites were added in the 5′ and 3′ end of the chimeric gene to the N- and C-terminal sequence, according to the Webcutter2 server. Furthermore, 6 poly-histidine (His) residues were appended to the C-terminal region at the ends of the sequence for more valid affinity purification. At last, the ultimate vaccine was cloned into the pET-22b (+) plasmid using SnapGene software (https://www.snapgene.com/try-snapgene/) to prove the expression procedure (32).
4. Results
This study is an experimental study. Figure 1 illustrates the procedure of vaccine design.
4.1. Multi-epitope Vaccine Conception
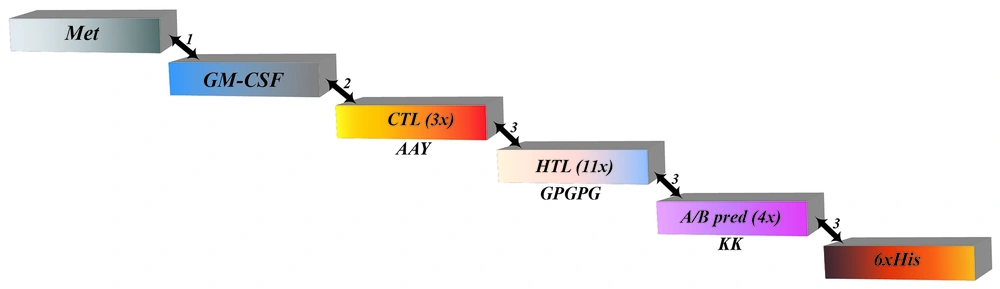
The best 33 epitopes revealed a score below 0.4 (Appendices 1 and 2 in Supplementary File) that produced VaxiJen antigenicity analysis. The HTL epitopes were evaluated for HLA-DRB1 (*03: 01, *07: 01, and *15: 01), HLA-DRB3 (*01: 01 and *02: 02), HLA-DRB4*01: 01, and HLA-DRB5*01: 01 subtypes. The highest binding capability was the main point to classify the lowest scores of the best epitopes. Due to positive IFN-γ and IL-4 inductions, the best candidate was selected (Appendix 1 in Supplementary File). The CTL epitope (9-mer) prediction was performed via the NetCTL-1.2 server and IEDB (Appendix 2 in Supplementary File). We utilized both BepiPred and ABCpred servers to recognize linear B-cell epitopes. All predictions of these servers were analyzed, and merely the overlapping epitopes were chosen to develop the vaccine. The 4 common epitopes in BepiPred and ABCpred contained prediction scores with a range of 0.5 to 1 and 0.52 to 0.93 were selected. Out of the whole, 18 epitopes were chosen as final components (Table 1). To create the subunit vaccine, we applied a suitable adjuvant and linkers (Figure 2). The N-terminal region begins with methionine (Met) attached to GM-CSF as an adjuvant by the EAAAK linker. Next, between the adjuvant and the primary CTL epitope, the GGGS linker was inserted. Three CTL epitopes attached using AAY linkers were joined to the primary HTL epitope by the HEYGAEALERAG linker. The GPGPG linker assisted the connection of all 11 HTL epitopes. Moreover, 4 ABCpred and BcePred epitopes were linked via the KK linker. Ultimately, the C-terminal region included a poly-His tail connected via the HEYGAEALERAG linker. The length of the ultimate vaccine structure was 481 amino acids.
| CTL Epitope | VaxiJen | HTL Epitopes | VaxiJen | ABCpred/BcePred | VaxiJen |
|---|---|---|---|---|---|
| LTDDVELAL | 0.4731 | GGRVNAVGYGESRPV | 1.1297 | VELALSYGEYHDVRGT | 1.0432 |
| VRDVLVNEY | 0.5999 | VEGGRVNAVGYGESR | 1.2633 | VGYGESRPVADNATAE | 0.7755 |
| AQARADEAY | 0.8764 | EGGRVNAVGYGESRP | 1.1695 | LDAIYHFGTPGVGLRP | 0.5930 |
| TPGVGLRPYVSAGLA | 1.2267 | TGCSSHSKETEARLTA | 1.8168 | ||
| GCSSHSKETEARLTA | 1.8777 | ||||
| ATGCSSHSKETEARL | 1.9224 | ||||
| LATGCSSHSKETEAR | 1.8273 | ||||
| DVESLRVERLAAPAA | 0.7976 | ||||
| GGSAVGGLARLGAKI | 1.0905 | ||||
| ARLGAKIGGKAAEMT | 1.5589 | ||||
| RLGAKIGGKAAEMTA | 1.5994 |
Schematic representation of the multi-epitope-designed vaccine construct. The following linkers connected epitopes: (1) EAAAK, (2) GGGS, and (3) HEYGAEALERAG. The AAY and GPGPG linkers, shown in the figure, connected cytotoxic T lymphocyte and helper T lymphocyte epitopes, respectively (Abbreviations: Met, methionine; GM-CSF, granulocyte-macrophage colony-stimulating factor; CTL, cytotoxic T lymphocyte; HTL, helper T lymphocyte; His, histidine).
4.2. Assessment of Allergenicity, Antigenicity, and Physiochemical Characteristics of the Vaccine
The AlgPred and AllerTOP servers found out the allergenic constitution of the vaccine. The vaccine at a threshold value of 0.4 was nonallergic with 94.18% accuracy. Furthermore, the antigenic feature of the designed vaccine structure at the default threshold value of 0.4 was 0.8421 (probable antigen), which indicated that the protein was a favorable antigen.
Besides, the designed vaccine construct included 481 amino acids. The theoretical isoelectric point and molecular weights were 6.24 and 50.83 kDa. The vaccine included 52 positively and 59 negatively charged residues. In addition, the vaccine-designed construct was formed of 7049 atoms with the chemical formula C2212H3473N649O700S15. The measured instability index was 31.92, which proved that it was stable. Moreover, the evaluated half-life was 30 hours in vitro, greater than > 20 hours in yeast and > 10 hours in E. coli. It was noted that the high thermostability was approved via the aliphatic index (69.79). According to the GRAVY value, which was shown to be -0.414 in this study, it revealed the hydrophobicity of the protein.
4.3. Analyses of Immune Response
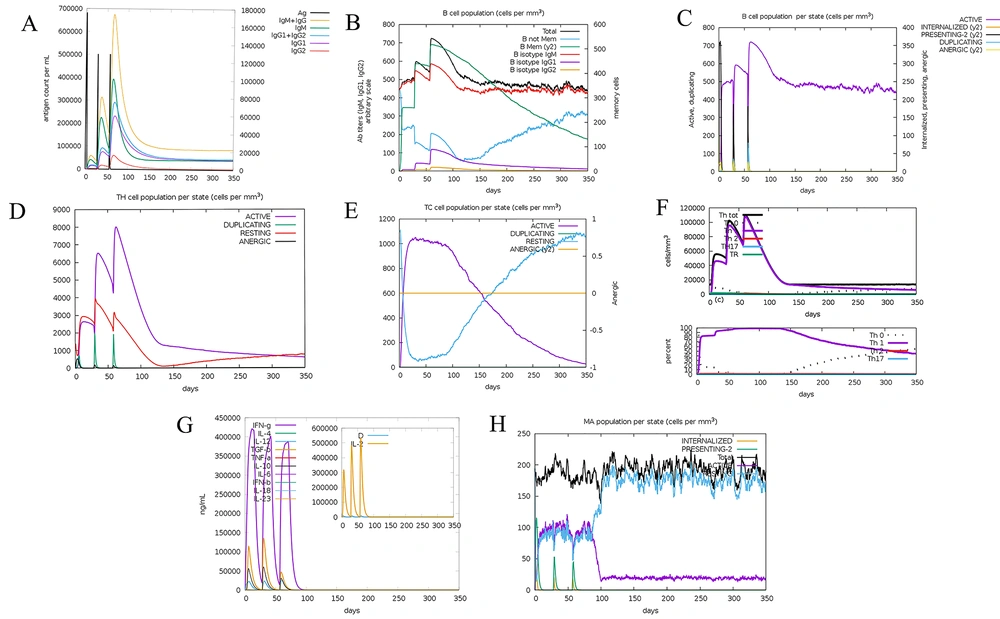
It was determined that the results of the C-ImmSim server were consistent with previous laboratory studies that announced the immune responses in P. aeruginosa infections. High titers of immunoglobulin (G, M, G1, and G1+G2) antibodies were recognized, resulting in a decrease in antigen concentration (Figure 3A). Therefore, an extension in the population of active B cells was identified, and memory B cell was specially raised (Figures 3B and C). A related behavior was recognized for HTL and CTL (Figures 3D and E), which indicated that the population of T cells was extremely responsive since the memory was produced. Through multiple exposures, IL-12, the concentration of IFN-γ, and the population of Th1 were sustained at significant levels (Figures 3F and G). Among innate immune cells, the outcomes showed elevated macrophage activity (Figure 3H). Therefore, these results showed that our vaccine design could efficiently provide the basis of immunity against P. aeruginosa infections and trigger the immune response.
Immune simulation results by C-ImmSim. A, Antigen and immunoglobulins; B, B-cell population; C, B-cell population per state; D, T helper cell population per state; E, T cytotoxic cell population per state; F, T helper cell classes’ switch; G, production of cytokine and interleukins; and H, macrophage population per state.
4.4. Secondary, Tertiary Recognition, and Verification of the Chimeric Vaccine Construct
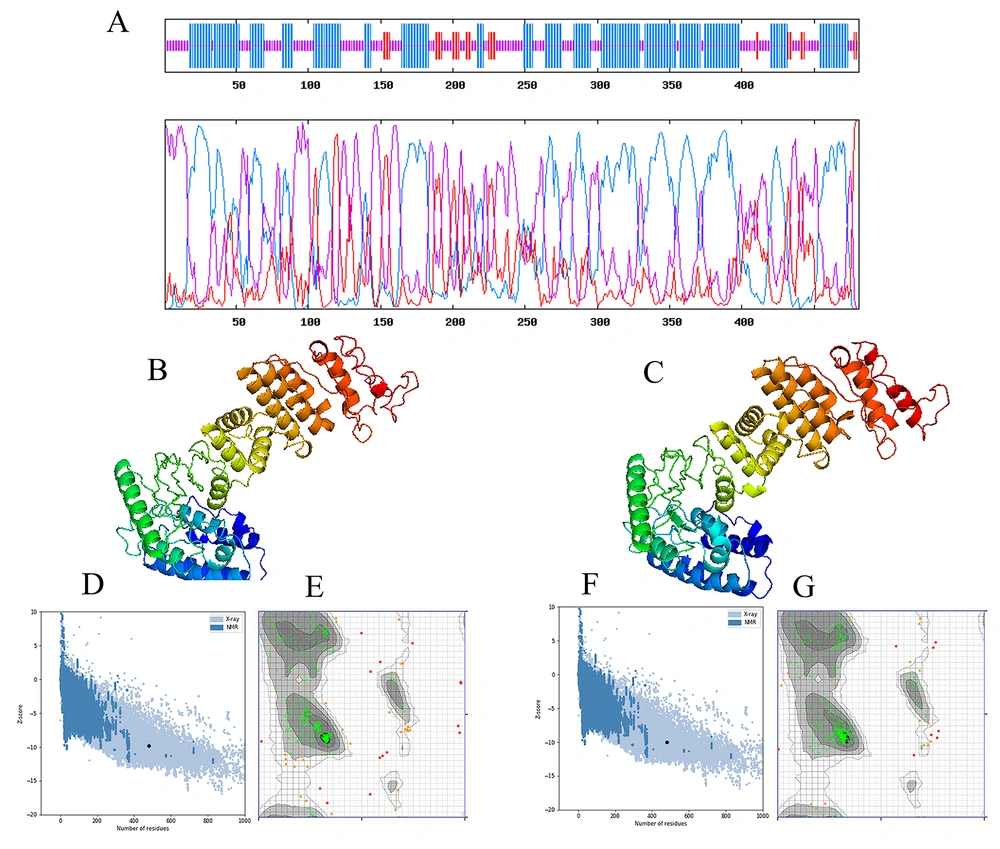
A high percentage of random coils and helices in the second construct of the multi-epitope protein was predicted using GOR V demonstrated a 481-amino-acid-long construct, including 7.07% of strands, 59.39% of helices, and 40.54% of random coils, which consisted of the formation of stable antigenic epitopes (Figure 4A).
A, graphical outcomes for secondary structure prediction of multi-epitope vaccine by the GOR V server. Then, the multi-epitope vaccine modeling and refinement were carried out; B, the initial tertiary structure was produced by the I-TASSER server presenting; D, the z-score of the initial model was -9.81; E, 88.030% of the residues in favored regions, 8.229% in allowed regions, and 3.741% in disallowed regions; C, the refined tertiary structure was achieved by the GalaxyRefine server presenting; F, the z-score of the refined model was -9.96; G, 94.26, 3.741, and 1.995% of the residues in favored, allowed, and disallowed regions, respectively.
According to a de novo template modeling-helix bundle topology, from 10 cases of the proposed models with a template modeling-score below 0.4, the best model indicated an approximately correct topology of the template modeling-helix assembly was chosen. This structure’s template modeling score and the root-mean-square deviation (RMSD) were 0.477 and 6.65. The template modeling score determines the similarity between 2 structures by a score between 0 and 1, in which 1 presents a good match with 2 structures (Figures 4B and C). After determining the most suitable model, a refinement process was performed via the GalaxyWeb server to enhance structure quality. Consequently, the server produced 5 refined models (see Appendix 3 in Supplementary File). We analyzed initial and refined models to perceive their differences and select the best model. The structural validation and reliability of the predicted model of the chimeric vaccine were carried out by different online tools, such as ERRAT, Verify-3D, Ramachandran plot (Figure 4D - G), PROCHECK, and RAMPAGE (Table 2).
| Models | Ramachandran Plot | Z-Score | ERRAT | Verify 3D (%) | ||
|---|---|---|---|---|---|---|
| Favored | Allowed | Disallowed | ||||
| Initial | 88.030 | 8.229 | 3.741 | -9.81 | 72.45 | 81.876 |
| Refined | 94.26 | 3.741 | 1.995 | -9.96 | 75.05 | 87.11 |
a All the results confirmed that the refined model was better than the initial model.
4.5. B-Cell Epitope Prediction
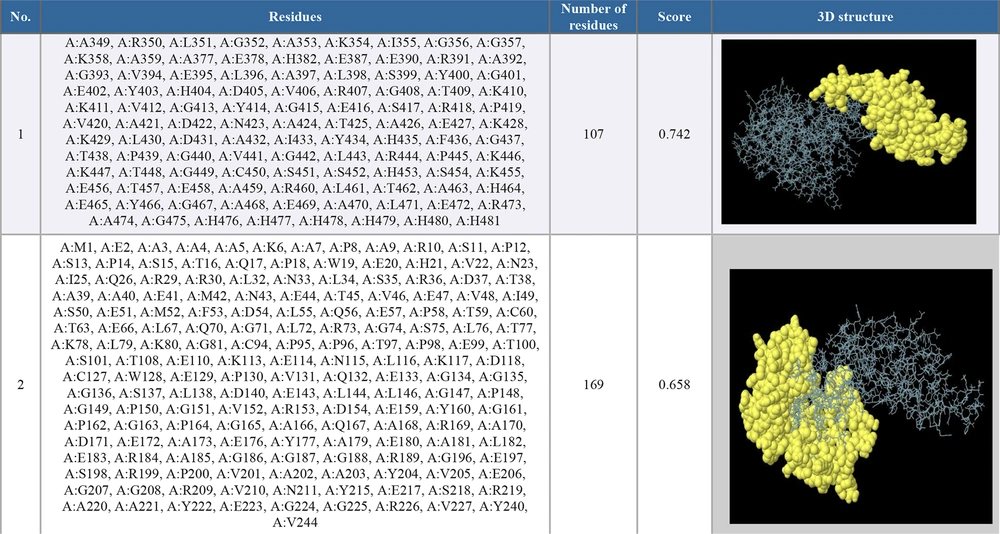
The selected PDB format of the chimeric vaccine was entered into the ElliPro server. We determined the minimum score as default (0.5) to select the epitope prediction parameter. Two epitopes were predicted with scores ranging from 0.658 to 0.742 (see Figure 5).
4.6. Investigation of Stability Between Vaccine Construct and TLR4
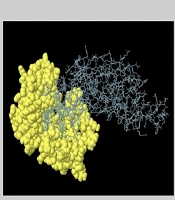
For further assessment of the binding affinity between homology modeling of the construct and TLR4, molecular docking accomplished by the ClusPro 2.0 server produced 30 clusters of protein-receptor complexes, accompanied by respective free binding energy, as an output. The best-weighted score of the lowest energy and the center score were -1132.4 and -927.1. As our investigations, cluster 1 was adopted, which accurately occupied the receptor and created a stable ligand-receptor complex (Figure 6A).
A, the interaction between toll-like receptor 4 (in light magenta color) and multi-epitope vaccine construct (yellow). The interaction was visualized with PyMol. Molecular dynamics between the chimeric vaccine and toll-like receptor 4 was administered utilizing the ClusPro server, and the product demonstrated the formation of a stable complex. In addition, the results of the molecular dynamics simulation study of multi-epitope vaccine and toll-like receptor 4 docked complex were illustrated. Here, B, normal mode analysis mobility; C, deformability; D, B-factor; E, eigenvalues; F, variance (red color illustrates individual variances, and green color shows cumulative variances); G, covariance map [correlated (red), uncorrelated (white), or anti-correlated (blue) motions]; and H, elastic network (darker gray regions demonstrate more stiffer regions).
The results of the MD simulation of the multi-epitope vaccine and TLR4 docked complex are demonstrated in Figure 6B. MD simulation of proteins provides an apparent measurement of their atoms and molecules’ physical and stability movements. Therefore, the simulation was performed to illustrate the relative strength of the multi-epitope vaccine protein. The deformability and B-factor graphs of the vaccine/TLR4 complex represented the peaks describing the moderate level of deformability and precise correlation between normal mode analysis (NMA) and the PDB field of the docked complex (Figures 6C and D). The eigenvalue of the complex is represented in Figure 6E. The multi-epitope and TLR4 docked complex produced a great eigenvalue of 1.094677e-05. Individual and cumulative variances are represented by red and green colored bars in the variance graph (Figure 6F). Figure 5G depicts the covariance map of the docked complex (red color, the correlated; white color, the uncorrelated; and blue color, the anti-correlated motions between residues). The elastic map of the docked complex, in which darker gray regions represent stiffer regions, points to the association between the atoms (Figure 6H).
4.7. Investigation of Codon Optimality, Bias, and Usage in the Translation of Chimeric Vaccine
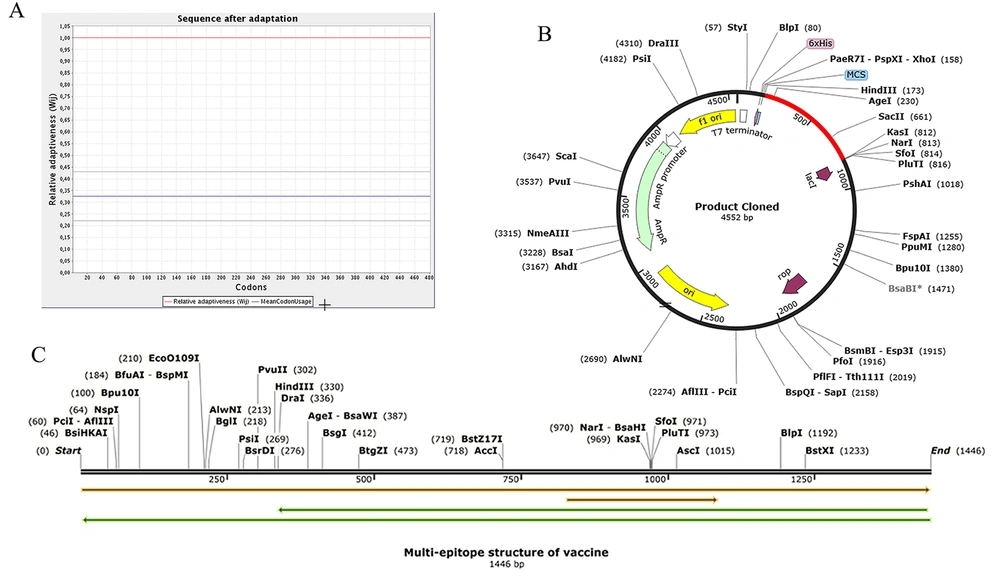
Whenever the codon usage of the host varies from the organism, codon adaptation is utilized to achieve significant expression in the host. The JCat was applied to determine codon optimization for maximal protein expression in E. coli (K-12 strain). It was noted that the length of the produced complementary DNA (cDNA) sequence was 1446 nucleotides. The CAI value was detected to be 1.0, revealing high gene expression (the optimum range is 0.8 - 1.0). Moreover, the average GC content (the optimal percentage is between 30 - 70%) of the improved sequence was 54.12% (Figure 7A). The predicted DNA sequence of the multi-epitope vaccine candidate (Figure 7C) was inserted into the pET-22b (+) vector plasmid between the HindIII and KasI restriction sites. The ultimately constructed vector is represented in Figure 7B.
Codon adaptation and in silico cloning. A, The results of the codon adaptation investigation of the best multi-epitope vaccine; B, the cloning of the gene sequence of vaccine constructs into pET-22b (+) vector, presenting the region of choice in red color surrounded between HindIII (173) and KasI (812), while the vector was displayed in black lines; C, the construct of multi-epitope vaccine.
5. Discussion
The current investigation was designed to build potential vaccines against P. aeruginosa, causing virulent and persistent infections despite antibiotic treatment. Recent studies have shown an increasing trend in the occurrence of multidrug-resistant (MDR) P. aeruginosa strains, which significantly limits the availability of chemotherapeutic agents for the treatment of patients and complicates the management of the disease (33, 34). With the emergence of MDR strains, P. aeruginosa-related infections are notoriously challenging to treat; hence, a possible vaccine was predicted in this study to combat this pathogenic microorganism. The bacteria’s 3 candidate proteins (OprF, OprI, and PopB) were recognized and chosen from the National Center for Biotechnology Information (NCBI) database to produce the vaccine construction. Since these antigenic proteins involve several crucial functions of P. aeruginosa and could provoke immune response effectively, these sequences were chosen for further analysis. Fito-Boncompte et al. in 2011 proved that OprF had a prominent role in cellular adhesion and production of virulence-related factors, including biofilm (35). A study showed that immunization with OprF/OprI and flagellin B was effective against infection generated by mucoid and non-mucoid strains of P. aeruginosa in animal models (36). In a previous study, immunization with highly conserved PopB, a component of the type III secretion system, evoked Th17 responses and intensified P. aeruginosa from the spleen and lung after challenge in an animal model (37). Following our previous study, OprF/I and PopB could simultaneously make antibody-independent and antibody-dependent protection. Therefore, in this study, we determined to work on these antigenic epitopes to develop a more effective vaccine.
The most important factor in improving computational analysis is the structural study of the vaccine candidate, predictions of antigenicity, investigating epitopes regarding a vast diversity of HLAs, and assessment of interactions with human receptors (38). The principal feature for the vaccine approach is the determination of corrected CTLs, HTLs, and B-cells epitopes, which can produce neutralized antibodies against the pathogenic microorganism and provoke the Th cells and CTL response (39).
The connection between epitopes produced by applying linkers can reduce the probability of junctional antigens generation and improve antigen-presenting cells (40). The structural flexibility and rigidity of a suitable vaccine construct are relevant to choosing linkers. Regarding the study by Rahmani et al. (41), we designed a vaccine peptide with the following linkers: EAAAK, GGGS, GPGPG, HEYGAEALERAG, AAY, and KK. EAAAK was applied between the adjuvant and Met to produce rigidity and decrease possible interference of other protein regions (42). GGGS was used between the adjuvant and CTL epitope to make better flexibility. The proteasomal and lysosomal systems (AAY and HEYGAEALERAG) used GPGPG linkers as cleavage sites to provoke HTL immune response (43). Ultimately, B-cell epitopes were connected with the KK linker to maintain their distinct immunogenic activity (44). This result illustrated that the selection and distribution linkers were suitable.
Various studies related to promising vaccine antigens for P. aeruginosa have indicated that Th1 and IFN-γ responses are linked to protection against this bacterial infection (45, 46). Concerning these observations, the choice of the GM-CSF adjuvant, as an additional component of the multi-epitope vaccine, seemed suitable. A study revealed that GM-CSF administration accompanied by a human immunodeficiency virus type 1 DNA vaccine increased the quality and quantity of vaccine immune T-cell responses. This event was carried out primarily via developing proliferating CD4+ T cells, which concurrently create IFN-γ, IL-2, and tumor necrosis factor-α (TNF-α) (47). Reali et al. noticed an improvement in the immune response following the local administration of GM-CSF in animal models (48). According to the result of the IFNepitope server analysis and IL4Pred, it was documented that IFN-γ-inducing epitopes and IL4-induced predicted could cause a long-lasting cellular response. The epitopes with high antigenic properties, low allergenicity, and non-toxicity were considered a vaccine candidate. Besides, the C-ImmSim server predicted a relevant antibody production immunoglobulin (G, M, G1, and G1+G2) after immunization, which is defined as necessary for fighting against Pseudomonas infection. These outcomes indicate the probability of this vaccine candidate to provoke an effective immune response and defend against the infection.
The characteristic physicochemical analysis was performed for the chimeric protein. The aliphatic index of the vaccine constructs, which depends on the relative volume involved via the aliphatic amino acids, valine, and alanine, had the highest value among these proteins (69.79). According to Kyte and Doolittle, the negative GRAVY value exhibits a hydrophilic protein, and the positive one expresses hydrophobic characteristics (49); our proteins had the highest GRAVY value of -0.414. Moreover, the half-life was 30 hours in mammalian reticulocytes, > 20 hours in yeast, and > 10 hours in E. coli; it had the highest theoretical PI of 6.24. Due to an instability index of 31.92, the vaccine was considered stable. Thus, the physicochemical property analysis exhibited quite good results.
The secondary structure analysis illustrated that the vaccine construct determined random coils (40.54%), alpha helices (59.39%), and extended strand (7.07%). These data revealed that this structure was stable with a random coil structure, facilitating identification by immune system agents. Next, the tertiary structure prediction of the chimeric vaccine was accomplished by applying the I-TASSER server. The GalaxyRefine server refined and improved the structural disturbance for the primary model. Then, the predicted 3D model validation using verified software revealed that 94.26% of residues were located in the favored region. The mentioned results confirmed that the predicted structure model was stable, profoundly ordered, and contained high features.
TLRs are among the most investigated pattern recognition receptor (PRR) families (50), which can identify explicitly bacterial lipopolysaccharide (LPS). Their activation significantly contributes to the construction of pro-inflammatory cytokines, for instance, TNF, IL-6, and chemokines. The molecular docking was performed to recognize whether all the epitopes could connect with their respective MHC I and II alleles or not. The results manifested interactions between the multi-epitope-designed vaccine and receptor, using ClusPro 2.0, relying on the lowest energy score (-1132.4) carried out thoroughly. Then, the MD simulation investigation, administered by the online tool iMODS, showed that the TLR4/multi-epitope docked complex was stable, including a valid eigenvalue of 1.094677e-05.
Codon usage and in silico cloning practices were conducted with the predicted CAI value of 1.0. The result of the DNA sequence showed that it contained a high value of favorable codons and a considerate amount of GC content of 54.12% in E. coli strain K12. Ultimately, the pET-22b (+) vector, containing the ultimate vaccine, was inserted to construct a more efficient vaccine candidate in E. coli cells. Peluso et al used both recombinant His-conjugated OprF (acquired from cloning the oprF gene into the pET-28a expression vector) and native OprF (isolated and purified from PAO1). They urged that DCs induced with these antigens could provoke Th1 cells and cause resistance to infection caused by either a clinically isolated mucoid or the PAO1 strain (51). This result is consistent with the result obtained by Wu et al who indicated a high level of IL-4, IL-17, and IFN-γ in animal models with the PopB and curdlan adjuvant compared to the control group (37). Moreover, our result was consistent with Hu et al, showing that TLR4 was crucial in host resistance to P. aeruginosa, and its deficiency led to increased polymorphonuclear (PMN) infiltration and pro-inflammatory cytokine production and a susceptible phenotype (52).
Altogether, the present study provides a snapshot of a multi-epitope vaccine against P. aeruginosa. We conceive that this research can be used as a framework for future studies to improve different types of P. aeruginosa vaccine. However, more in vitro and in vivo studies are needed to confirm the findings of this study.
5.1. Conclusions
The Pseudomonas-host relationship complexity in each stage of infection is not apparent, preventing the development of an approved vaccine. In the current study, we investigated a multi-epitope chimeric vaccine using different computational studies based on protective antigens of P. aeruginosa (OprF, OprI, and PopB). A reasonably chimeric-designed vaccine is required to develop both innate and adaptive immunity according to the presence of several T-cell and B-cell epitopes, IL-4 and IFN-γ inducing epitopes, and indicated stability-binding affinity with the immune-receptor TLR4. Hence, to generate potential vaccines against P. aeruginosa, we suggest that specialists construct and purify this chimeric vaccine and investigate the vaccine efficacy before and after challenge into appropriate models.
![A, the interaction between toll-like receptor 4 (in light magenta color) and multi-epitope vaccine construct (yellow). The interaction was visualized with PyMol. Molecular dynamics between the chimeric vaccine and toll-like receptor 4 was administered utilizing the ClusPro server, and the product demonstrated the formation of a stable complex. In addition, the results of the molecular dynamics simulation study of multi-epitope vaccine and toll-like receptor 4 docked complex were illustrated. Here, B, normal mode analysis mobility; C, deformability; D, B-factor; E, eigenvalues; F, variance (red color illustrates individual variances, and green color shows cumulative variances); G, covariance map [correlated (red), uncorrelated (white), or anti-correlated (blue) motions]; and H, elastic network (darker gray regions demonstrate more stiffer regions). A, the interaction between toll-like receptor 4 (in light magenta color) and multi-epitope vaccine construct (yellow). The interaction was visualized with PyMol. Molecular dynamics between the chimeric vaccine and toll-like receptor 4 was administered utilizing the ClusPro server, and the product demonstrated the formation of a stable complex. In addition, the results of the molecular dynamics simulation study of multi-epitope vaccine and toll-like receptor 4 docked complex were illustrated. Here, B, normal mode analysis mobility; C, deformability; D, B-factor; E, eigenvalues; F, variance (red color illustrates individual variances, and green color shows cumulative variances); G, covariance map [correlated (red), uncorrelated (white), or anti-correlated (blue) motions]; and H, elastic network (darker gray regions demonstrate more stiffer regions).](https://services.brieflands.com/cdn/serve/3170b/1f713c28d65f9ddaf16133620919862e4b50c593/archcid-118243-i005-F6-preview.webp)