1. Background
Infection caused by SARS-CoV-2 can lead to significant procoagulant events, sometimes involving life-threatening pulmonary thromboembolism (PE) (1). Several abnormalities have been described in coagulation parameters, which are predictors of poor prognosis in patients with COVID-19 and PE (2). Due to the lack of large prospective studies, little is known about the pathogenesis underlying PE caused by COVID-19 (3, 4).
The PE diagnosis is challenging in COVID-19-affected patients. Prolonged immobilization and hypercoagulable state are considered the predisposing factors for the PE onset. Viral particles provoke a systemic inflammatory response, which, in turn, leads to a violation of the balance between the procoagulant and anticoagulant state in the body. Blood clotting is to prevent the loss of blood and immune components. Thrombosis could reduce the entry of microorganisms into the blood. Endothelial dysfunction is blamed as a possible provoker of microthrombosis. The resultant dysfunction makes endothelial cells lose their basic properties, such as vasodilation, antiplatelet activity, and fibrinolysis (1). Additional conditions complicating the diagnosis are the presence of risk factors for PE in almost all patients with COVID-19 and the overlap of the clinical presentation between PE and COVID-19. Understanding these factors would lead to the early diagnosis and prevention of potentially fatal complications by applying timely and appropriate treatment. In the treatment of COVID-19 patients complicated by PE, the use of systemic fibrinolysis or catheter-targeted therapy should be limited to those strictly indicated (1, 5).
2. Objectives
We aimed to find the indicators predicting the presence of PE in patients with acute or post-acute COVID-19 conditions.
3. Methods
3.1. Treatment of COVID-19 Patients in Heart and Brain Centre of Excellence, University Hospital, Pleven, Bulgaria
The COVID-19 department of Heart and Brain Centre of Excellence, University Hospital, Pleven, Bulgaria, was opened in November 2020, and by April 2021, it already had 94 beds, including 20 intensive beds equipped with mechanical ventilation. All patients hospitalized with COVID-19 pneumonia received a therapeutic dose of anticoagulant and a prophylactic dose of antiplatelet agent during treatment. At their discharge, an antiplatelet agent was recommended for one month unless there was an indication for more prolonged use.
3.2. Study Group
A single-center study was conducted at the Heart and Brain Hospital, Pleven, from December 2020 to February 2021. It included 27 consecutively hospitalized patients with recent pneumonia caused by COVID-19 and clinical presentation corresponding to PE. The inclusion criteria were patients aged ≥ 18 years with active or experienced COVID-19 pneumonia, clinical, laboratory, and diagnostic criteria for PE, and no allergy to iodine-containing contrast agents, who confirmed their participation by written consent. The exclusion criteria were refusal to participate in the test and allergy to contrast. The cohort was divided into two groups with and without a definitive diagnosis of PE, proven by CT pulmoangiography. During COVID-19 treatment, all patients received a prophylactic dose of the anticoagulant and antiplatelet drug. The treatment management of patients diagnosed with PE was in line with the European Society of Cardiology recommendations. Due to the higher risk of bleeding, catheter-targeted thrombolysis with Actilyse was performed according to a protocol. For this purpose, the right femoral vein was used for vascular access, and 15 - 20 mL of Actilyse was injected into the affected branch of the pulmonary artery or bilaterally using a pigtail catheter.
3.3. Statistical Analyses
Statistical analyses were performed using SPSS for Windows version 20.0 statistical software. Continuous variables are presented as mean ± standard deviation (SD). The categorical variables were presented as percentages. Comparisons of continuous variables between the two groups were made with the Mann-Whitney Test. Fisher's exact test evaluated the relationship between diagnosis and categorical variables. Receiver operating characteristic analysis (ROC) was used to determine the diagnostic capabilities of D-dimer. Sensitivity (Se), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) were calculated at the D-dimer cutoff value of 1,032 ng/m. A two-tailed P-value of < 0.05 was considered statistically significant.
3.4. Ethical Considerations
All patients signed informed consent forms for pulmoangiography, fibrinolysis, and personal data analysis. The study protocol followed the Declaration of Helsinki.
4. Results
Our results showed that eight patients from the cohort had PE, and 19 had no evidence of PE. The cohort's mean age was 65 years, and 18 patients were women. The two groups did not differ significantly in age and sex distribution (Table 1).
| Variables | Patients with Active or Experienced COVID-19 Pneumonia Without PE Definitive Diagnosis; n = 19 | Patients with Active or Experienced COVID-19 Pneumonia with PE Definitive Diagnosis; n = 8 | P-Value |
|---|---|---|---|
| Age (y) | 65.37 ± 10.57 | 65.63 ± 11.40 | 0.710 |
| Female | 13 (68.4) | 5 (62.5) | 1.000 |
| VTE | 1 (5.3) | 1 (12.5) | 0.513 |
| Recent trauma/surgery | 1 (5.3) | 1 (12.5) | 0.513 |
| Active neoplastic process | 1 (5.3) | 1 (12.5) | 0.513 |
| Previous PE | 1 (5.3) | 0 (0.0) | 1.000 |
| Overweight/obesity | 14 (73.7) | 7 (87.5) | 0.277 |
| Heart rate ≥ 75/min | 13 (68.4) | 8 (100) | 0.153 |
| Symptoms of HF | 2 (10.6) | 3 (37.5) | 0.169 |
| SatO2 at admission (%) | 90.21 ± 7.40 | 89.00 ± 3.78 | < 0.001 |
| ICU stay | 4 (21.1) | 1 (12.5) | 1.000 |
| D-dimer (ng/mL) | 1546.00 ± 2082.13 | 6489.75 ± 6127.30 | 0.021 |
| hsCRP (mg/L) | 41.48 ± 50.37 | 45.31 ± 41.65 | 0.832 |
| Leu (× 109 g/L) | 7.75 ± 2.42 | 9.80 ± 3.25 | 0.123 |
| Lym (× 109 g/L) | 1.25 ± 0.71 | 1.45 ± 1.20 | 0.873 |
| Plt (× 109 g/L) | 327.95 ± 164.48 | 249.13 ± 80.92 | 0.265 |
| Shortness of breath after experiencing PE | 14 (73.7) | 4 (50.0) | 0.375 |
| Edema after experiencing PE | 3 (15.8) | 2 (25.0) | 0.616 |
| Cough after experiencing PE | 4(21.1) | 2 (25.0) | 1.000 |
a Values are expressed as No. (%) or mean ± SD.
The two groups did not differ significantly according to demographics and baseline characteristics. In the context of the disease, increased inflammation markers were observed in all patients. Blood oxygen saturation was markedly lower in the group with definite PE. Notably, shortness of breath and fatigue persisted after controlling the disease in most patients.
Statistically significant differences in electrocardiographic findings were observed between the two groups. In the group without PE, 18 (94.7%) patients had no evidence of S-wave greater than 1.5 mm in leads I and aVL. On the other hand, in the group diagnosed with PE, this ECG criterion was not present in three (37.5%) patients but present in five (62.5%) (P = 0.004). Similar ratios were found in terms of the presence of Q-wave in leads III and aVF. In patients without PE, 18 (94.7%) did not have this ECG sign, while it was present in half of the patients with PE (P = 0.017).
Statistically significant differences between the two groups were observed regarding the ratio of RV/LV diameters ≥ 1.0 (P = 0.001). In patients without PE, no one had an increase in the ratio ≥ 1 in favor of the right ventricle, while in the group of patients with massive form, five (62.5%) had the ratio of RV/LV diameters ≥ 1.0, and three (37, 5%) did not have. In patients without PE, no one had right ventricular dysfunction, while in patients with massive form, five (62.5%) had right ventricular dysfunction, and three (37, 5%) did not have (P = 0.001). The RV/LV diameter ratios ≥ 1.0 and right ventricular dysfunction showed Se of 62.5%, Sp of 100%, PPV of 100%, and NPV of 86.4% to verify the PE diagnosis.
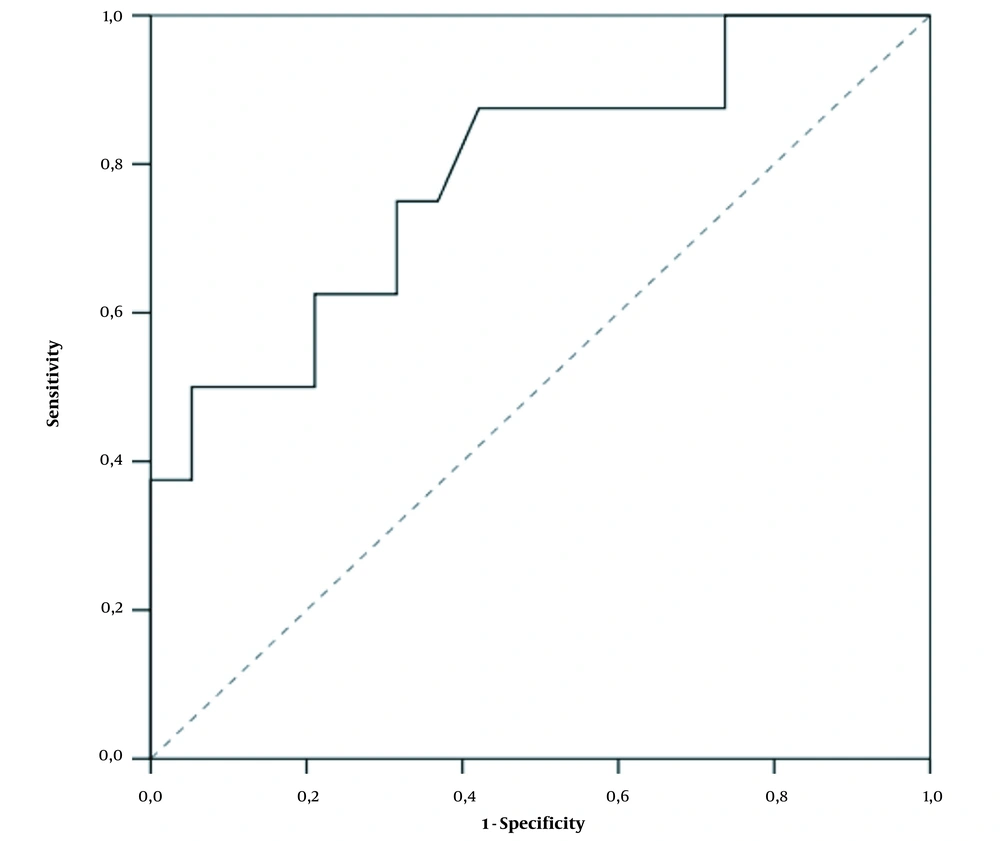
D-dimer values differed significantly between the two groups. In patients without PE, the mean D-dimer value was 1,546 (109 - 8840) ng/mL, while it was 6489.75 (570 - 17051) ng/mL in those with PE (P = 0.021). Our laboratory uses the normal range upper limit of 500 ng/mL. As a result of the ROC analysis, we found that the D-dimer cutoff value of 1,032 ng/mL (2.064 times above the normal range upper limit) had an optimal Se of 87.5%, Sp of 57.9%, PPV of 46.7%, and NPV of 91.7% for PE diagnosis (P = 0.021) (Figure 1).
Regarding D-dimer as a binary variable (cutoff 1,032 ng/mL), we found that in the group without PE, 11 (57.9%) patients had D-dimer ≤ 1,032 ng/mL, while eight (42.1%) patients had D-dimer > 1,032 ng/mL. Of the patients with massive PE, only one (12.5%) patient had D-dimer ≤ 1,032 ng/mL, and the remaining seven (87.5%) patients had values > 1,032 ng/mL (Fisher`s exact tests, P = 0.043).
When performing binary logistic regression, some ECG criteria, including S-wave over 1.5 mm in leads I and aVL (P = 0.007), Q-wave in leads III and aVF (P = 0.020), and D-dimer as a quantitative variable (P = 0.025) proved to be independent predictors of PE.
5. Discussion
From our results, we found that ECG and EchoCG criteria remain the predictors of PE. The D-dimer cutoff with optimal Se, Sp, PPV, and NPV for PE diagnosis was two times higher than the normal range upper limit, with high Se and NPV.
The PE diagnosis is challenging in COVID-19-affected patients. The incidence of this complication is 1.9 - 8.9% in hospitalized patients. Critically ill patients admitted to intensive care units have the highest risk of developing PE, up to 26.6% (4, 6, 7). Prolonged immobilization and hypercoagulable state are considered the predisposing factors for PE onset. The hypercoagulable state was confirmed by Tang et al., who demonstrated that higher levels of D-dimer, fibrinogen, prolonged thromboplastin time, prothrombin time, and INR were predictors of poor prognosis in patients affected by SARS-CoV-2 (1, 8). Viral particles provoke a systemic inflammatory response, which, in turn, leads to a violation of the balance between the procoagulant and anticoagulant state in the body. The immune and coagulation systems are closely linked. Blood clotting is to prevent the loss of blood and immune components.
On the other hand, thrombosis could reduce the entry of microorganisms into the blood. In addition, the constituents of the platelets themselves have antimicrobial activity (1, 9). Therefore, the body seeks to limit the viral load through thrombosis. Deep venous thrombosis and other sources of non-venous thromboembolism have not been systemically detected in COVID-19 patients complicated by PE. Endothelial dysfunction is blamed as a possible provoker for the development of microthrombosis (1, 9, 10). Endothelial cells represent nearly one-third of the cells in the bronchoalveolar tree. As a result of the dysfunction, they lose their basic properties such as vasodilation, antiplatelet activity, and fibrinolysis. The endothelial cells themselves have receptors for SARS-CoV-2, namely angiotensin-converting enzyme-2 receptors, which facilitate the penetration of viral particles. Several cytokines released due to a systemic inflammatory response lead to endothelial cell apoptosis (1, 11).
Another predisposing factor for the hypercoagulable state in the body is hypoxia, which increases the viscosity of the blood. Several risk factors underlie the possibility of developing PE, such as age, obesity, family history of PE, heart and respiratory failure, pregnancy, stroke, trauma, surgery, and neoplastic diseases. It should be noticed that almost all patients with COVID-19, especially those hospitalized, have at least one risk factor and often multiple risk factors for venous thromboembolism. There are predisposing factors for PE in intensive care units, such as immobilization, sedation, and the use of central venous catheters (1, 12).
On the one hand, the clinical symptoms of COVID-19 patients, including shortness of breath, fever, and cough, are not specific for PE and are explained with the infection. In addition, SARS-CoV-2 binds to the angiotensin-converting enzyme 2 (ACE2) receptor and is expressed in brain neurons and glial cells. Therefore, the manifestation of cerebrovascular symptoms is also possible. A case of a patient with COVID-19 who developed a seizure and cerebral edema because of cerebral venous thrombosis has been described. A study in Italy found that the incidence of venous thromboembolism was 21% in 388 patients with COVID-19. A publication from Iran reported the presence of seizures in a patient with COVID-19 (13). There is still limited information on the occurrence of neurological symptoms in patients with COVID-19.
The study of the D-dimer does not give us objective information on this infection, and it is debatable to rely on it when considering the possibility of PE. In addition, the use of native computed tomography could not provide specific information for pulmonary thromboembolism. The possibility of developing contrast-induced nephropathy when performing computed tomography pulmoangiography should not be overlooked, especially in patients in shock with severe COVID-19 infection (4, 14-18). There are some logistical problems in computed tomography, including microcirculatory occlusions and thrombosis that this method cannot represent. Therefore, a stepwise clinical, laboratory, and radiological evaluation of COVID-19 patients should be performed when assessing the likelihood of PE.
Many data suggest that the prophylactic dose of unfractionated or fractionated heparin improves survival in some patients with criteria for sepsis-induced coagulopathy or very high D-dimer values (18). Although data are limited, recommendations have also been given for using a therapeutic dose of anticoagulants in hospitalized patients with COVID-19. However, the decision must be strictly individual and consider patients with multiple risk factors and critical conditions (1, 19, 20).
Algorithms have been developed to use D-dimer in all patients as a triage test to diagnose PE (20). At low values of 500 - 1000 ng/mL, PE is excluded without the need for CT pulmoangiography. Above these threshold values, it is necessary to implement the test. The threshold values of 500 - 1000 ng/mL in these algorithms have a 100% negative predictive value (21, 22).
According to Mouhat et al., the threshold above which PE can be most suspected in COVID-patients is 2590 ng/mL. They conducted a retrospective study of 162 patients with severe COVID-19 infection. In this study, this cutoff value had Se of 83.3%, Sp of 83.8%, PPV of 72.9%, and NPV of 90.5%. Therefore, it can be assumed that this threshold would lead to the omission of nearly 17% of pulmonary emboli cases (22, 23). In contrast to the above studies, we found that the D-dimer cutoff value of 1,032 ng/mL (2.064 times above the upper limit of the normal range) has optimal Se, Sp, PPV, and NPV for PE diagnosis (P = 0.021). Therefore, all these results suggest the need for further research and validation of a uniform cutoff value.
5.1. Limitations
Our limitations are related to the small selected group of patients, but our work continues to expand the cohort and present more detailed results.
5.2. Conclusions
Our results showed that against the background of acute and post-acute COVID-19 conditions, ECG and EchoCG criteria remain the predictors of PE. As for D-dimer values, we found that the cutoff value with optimal Se, Sp, PPV, and NPV for PE diagnosis is two times higher than the upper limit of the normal range, with high Se and NPV. We suggest that a higher D-dimer cutoff value be applied in COVID-19 and post-COVID-19 patients to confirm/dismiss the PE diagnosis.