1. Background
Coronavirus disease 2019 (COVID-19) is an emerging disease whose transmission type, treatment method, and side effects are unknown (1, 2). Considering that pregnant women and newborns are vulnerable groups, investigating the ways of transmission in pregnancy and fetal, maternal, and neonatal complications can help to reduce possible complications. One of the involved countries is Iran.
Pregnant women are more susceptible to respiratory infections and pneumonia, and physiological changes during pregnancy (e.g., raising the diaphragm, increased oxygen consumption, and edema of airway mucosa) make them more vulnerable to hypoxemia (2-5). According to studies, pregnant women are at higher risk of viral infections, such as influenza A, H1N1, SARS-CoV, MERS-CoV, and Ebola; they seem to manifest more severe clinical symptoms, including maternal mortality, spontaneous miscarriage, and preterm labor, compared to non-pregnant ones. However, there are limited data on the clinical manifestations of COVID-19 during pregnancy (6-9). A study enrolled nine near- and full-term pregnant women and reported that the disease is not probably transmitted from mother to fetus. They reported no severe involvement or maternal death (10). A case report of a 30-week pregnant woman suggested the possibility of disease transmission to the fetus and preterm labor (11).
2. Objectives
Hence, available data are limited, and larger-scale studies are required. Also, no study thus far has investigated near-term pregnant women. Due to the scarcity of data on the behavior of this virus in the first and second trimesters of gestation, the present study examined the disease at lower gestational ages and the possibility of vertical transmission to the fetus. We also investigated childbirth complications and the severity of the disease in mothers with possible underlying diseases, such as gestational diabetes, hypertension, heart disease, asthma, and hypothyroidism.
3. Methods
The protocol of the present case series study was approved by the Ethics Committee of Mazandaran University of Medical Sciences (ethics code: IR.MAZUMS..REC.1399.30). All the principles of research ethics were considered in the study. The study was performed from February 2019 to August 2020. A convenience sampling method was used to select the subjects. First, informed consent was obtained from the patients, and they were then examined for inclusion and exclusion criteria. Inclusion criteria included pregnant women above 18 years attending the hospital with a complaint of fever, shortness of breath, cough, diarrhea, myalgia, or decreased sense of smell, meeting one of the biochemical or imaging criteria in favor of COVID-19, and/or hospitalization due to the suspicion of COVID-19 pneumonia. Exclusion criteria were reluctance to participate in the study, lack of clinical and biochemical symptoms, no imaging findings in favor of COVID-19, and negative PCR results.
The researcher took the demographic information and medical history of eligible subjects. The author and one of the assistants collected data through a questionnaire. The collected information included demographic characteristics (maternal age, gestational age, number of pregnancies, weight, height, length of hospital stay, and history of exposure to the virus and travel), the severity of the disease, type of treatments received, underlying disease(s), childbirth complications, fetal infection, clinical and laboratory findings and symptoms related to pneumonia, maternal and fetal complications, and imaging findings. A throat swab sample for PCR was taken from eligible subjects after counseling with an infectious diseases specialist. According to the specialists' prescription, medication therapy was started for all the patients.
Out of 19 subjects enrolled, 17 had a positive PCR result for the throat specimen, of whom 11 also had positive PCR results for amniotic fluid taken at pregnancy termination by a gynecologist. The PCR was also performed for throat swab samples taken from babies. Termination of pregnancy, either natural delivery or cesarean section, was made in a separate room. The PCR for amniotic fluid was not carried out on five cases due to bloody or meconium-stained amniotic fluid. All surgeons, midwifery, and other operating and delivery room staff wore protective clothes, and health and safety protocols were observed. Pregnancy was terminated via cesarean section in most subjects, and only one had a vaginal delivery.
The neonatal transport team transported the baby according to health and safety protocols. Both mother and baby were separately monitored and cared for until recovery from the disease. The cause of pregnancy termination, gestational age at pregnancy termination, infant weight, Apgar scores at one and five minutes, and admission to the Intensive Care Unit (ICU) were recorded. Data were analyzed in SPSS version 23 using the chi-square test. Six weeks after delivery, the status of rehospitalization of the baby, breastfeeding status, rehospitalization of the mother due to COVID-19, and the state of depression of the mother were evaluated by a 21-item questionnaire over the phone.
4. Results
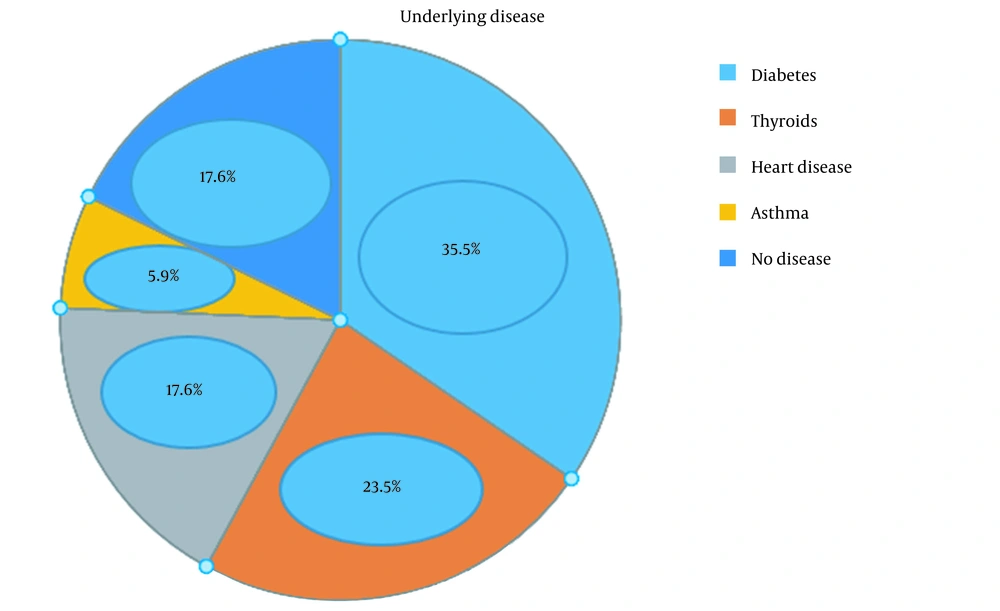
The research data are provided in five tables. Table 1 and Figure 1 shows the demographic and baseline characteristics with underlying disease and drug history, Table 2 the clinical symptoms of COVID-19 observed in the subjects, Table 3 the paraclinical symptoms reported in the participants, Table 4 the pregnancy outcomes, and Table 5 the PCR results (Table 6).
| Variables | Values |
|---|---|
| Age, y | 30 ± 6.1 |
| BMI, kg/m2 | 27.0 ± 5.14 |
| Gestational age, w | 33.9 ± 3.1 |
| Twin pregnancy | 2 (11.8) |
| Single pregnancy | 15 (88.2) |
| Nulliparous | 6 (35.3) |
| Multiparous | 11 (64.7) |
| Exposure to an infected person | 3 (17.6) |
| History of travel to infected areas | 0 (0.0) |
| History of other relevant infection | 2 (11.8) |
| History of husband's infection | 3 (17.6) |
| History of other relevant infection | 2 (11.8) |
| Prior use of face masks | 13 (76.5) |
| Transfer from another hospital | 7 (41.2) |
Abbreviation: BMI, body mass index.
aValues are expressed as Mean ± SD or No. (%).
| Variables | Values |
|---|---|
| Fever | 12 (70.6) |
| Headache | 1 (5.9) |
| Fatigue | 6 (35.3) |
| Cough | 10 (58.8) |
| Sore throat | 5 (29.4) |
| Dyspnea | 13 (76.5) |
| Diarrhea | 1 (5.9) |
| Lethargy | 10 (58.8) |
| Anosmia | 1 (5.9) |
| Chills | 3 (17.6) |
| Nausea | 2 (11.8) |
| Myalgia | 5 (29.4) |
| Body temperature, °C | 37.74 ± 1.08 |
| Pulse rates, min | 101.7 ± 15.62 |
| Diastolic blood pressure, mmHg | 5.8 ± 2.5 |
| Respiratory rates, min | 22.1 ± 6.1 |
aValues are expressed as Mean ± SD or No. (%).
| Variables | Values |
|---|---|
| White blood cells, mL | 8.48 ± 3.8 |
| Lymphocyte counts | 14.6 ± 4.7 |
| Hemoglobin | 10.64 ± 1.6 |
| RBC | 3.75 ± 0.4 |
| MCV | 83.26 ± 10.67 |
| MCH | 28.14 ± 3.7 |
| Platelet counts | 187 ± 58.16 |
| FBS | 91 ± 25.05 |
| Random blood sugar | 95.55 ± 31.44 |
| PT | 12.03 ± 0.29 |
| PTT | 31.15 ± 9.25 |
| INR | 1.0 ± 0.0 |
| ESR | 42 ± 22.83 |
| CRP | 31.53 ± 29.67 |
| AST | 44.73 ± 26.91 |
| ALT | 37.20 ± 39.94 |
| LDH | 358.83 ± 173.74 |
| O2 saturation | 94.52 ± 9.03 |
| NA | 126.7 ± 35.05 |
| D-Dimer | 6620 ± 0.0 |
| K | 3.7 ± .5 |
| CPK | 57.1 ± 47.4 |
| Troponin | 137.9 ± 379.8 |
| LL6 | 13.09 ± 4.1 |
| Feritin | 337.2 ± 347.1 |
| BLID | 0.33 ± .25 |
| BLIT | 0.93 ± .35 |
| UREA | 16.3 ± 8.9 |
| CR | 0.65 ± .176 |
| Procalsitonin | 0.60 ± 0.0 |
| CKMB | 28.4 ± 19.6 |
| Chest CT scan findings | 4 (23.5) |
| Mild infiltration | 7 (41.2) |
| Moderate infiltration | 4 (23.5) |
| Severe infiltration | 2 (11.8) |
| Normal |
aValues are expressed as Mean ± SD or No. (%).
| Variables | Values |
|---|---|
| Gestational age at admission (N = 17) | 33.9 ± 3.1 |
| Gestational age at termination | 35.6 ± 2.25 |
| Baby weight | 2764.11 ± 7769. |
| Duration of hospital admission, y | 5.8 ± 3.24 |
| ICU admission | 11 (64.7) |
| Route of pregnancy termination | |
| Vaginal delivery | 1 (5.9) |
| Cesarean section | 16 (94.1) |
| Delivery indications | |
| Midwifery indication | 4 (23.5) |
| Fetus indication | 5 (29.4) |
| Multidisciplinary team decision-making | 4 (23.5) |
| Term pregnancy | 4 (23.5) |
| Prior surgical history | 8 (47.1) |
| Critical effects | 12 (70.6) |
| Respiratory failure | 13 (76.5) |
| Renal complication | 2 (11.8) |
| Convulsion | 1 (5.9) |
| Decreased amniotic fluid | 2 (11.8) |
| Preeclampsia | 4 (23.5) |
| Maternal complications | 5 (29.4) |
| Pleural effusion | 1 (5.9) |
| Postpartum blues | 1 (5.9) |
| Maternal death | 1 (5.9) |
| Anti-COVID-19 drug intervention before termination | |
| Yes | 11 (64.7) |
| No | 6 (35.3) |
aValues are expressed as Mean ± SD or No. (%).
| Variables | Values |
|---|---|
| Baby weight | 2764.11 ± 776.9 |
| Preterm birth | 9 (52.9) |
| Neonate nasopharynx PCR test | |
| Positive | 2 (11.8) |
| Negative | 11 (64.7) |
| Not examined | 4 (23.5) |
| Amniotic fluid PCR test | |
| Positive | 6 (35.3) |
| Negative | 5 (29.4) |
| Not examined | 6 (35.3) |
| Umbilical cord blood PCR test | |
| Positive | 0.0 (0.0) |
| Negative | 3 (17.6) |
| Not examined | 14 (82.4) |
| Neonatal infection by COVID-19 | 2 (11.8) |
| Intact neonate | 15 (88.2) |
| Vertical transmission in neonate | |
| Yes | 2 (11.8) |
| No | 10 (58.8) |
| Unknown | 5 (29.4) |
| Neonate nutrition | |
| Breastfeeding | 15 (88.2) |
| Powder milk | 2 (11.8) |
| NICU admission | 10 (58.8) |
| Rehospitalization due to COVID-19 | 0.0 (0.0) |
aValues are expressed as Mean ± SD or No. (%).
| Variables | No. (%) |
|---|---|
| Maternal nasopharynx PCR test | |
| Positive | 13 (76.5) |
| Negative | 4 (23.5) |
| Vaginal secretion PCR test | |
| Positive | 0.0 (0.0) |
| Negative | 3 (17.6) |
| Not examined | 3 (17.6) |
The mean ± standard deviation (SD) of the subjects' age was 30 ± 6.1 years, with a body mass index of 27.0 ± 5.14 kg/m2 and gestational age of 33.9 ± 3.1 weeks. Of the 17 pregnant women hospitalized for COVID-19, two (11.8%) reported twin pregnancies and six (35.3%) first pregnancies, and three (17.6%) had a history of contact with an infected person. Five patients (29.4%) had severe, seven (41.2%) mild, and three (17.6%) moderate pneumonia.
The mean ± SD SPO2 was 94.52% ± 9.03% in the participants. Out of 17 hospitalized subjects, two (11.8%) developed severe lung involvement.
The mean ± SD gestational age was 35.6 ± 2.25 weeks at the termination of pregnancy and 5.8 ± 3.24 days for the hospital stay. Of 17 subjects hospitalized, 11 (64.7%) were admitted to the ICU, and 16 (94.1%) gave birth by cesarean section. Pregnancy was terminated due to fetal issues in five (29.4%) cases, and nine (52.9%) mothers gave birth to premature infants. Moreover, 13 (76.5%) subjects had respiratory problems, 12 (70.6%) critical complications, and four (23.5%) preeclampsia. One mother (5.9%) died from pulmonary edema caused by COVID-19, and another (5.9%) developed postpartum depression. Two (11.8%) infants were fed by a formula.
Ten cases (58.8%) of NICU hospitalization were found, and two infants (11.8%) were fed formula.
Two (11.8%) neonates with positive PCR results for nasal swab specimens, six (35.3%) with positive PCR results for amniotic fluid, two (11.8%) with COVID-19 infection, and two (11.8%) cases of vertical transmission were reported.
Based on the analysis, the prevalence of positive PCR results for amniotic fluid was significantly higher in preterm infants (P = 0.030), and mothers with positive PCR results for amniotic fluid had neonates with positive PCR results for nasal specimens, infection with COVID-19 (P = 0.012), positive vertical transmission, and admission to the NICU (P= 0.030).
Based on the analysis of results, the prevalence of positive PCR results for throat specimens was significantly higher among subjects aged 20-30 years (P = 0.037). The frequency of admission to the ICU was significantly higher in pregnant women with diabetes (P = 0.025). There was no significant relationship between the subjects' thyroid disease and ICU admission (P = 0.091).
5. Discussion
In the present study, six subjects reported a history of contact with an infected person, and no cases of travel to contaminated areas were reported. Some pregnant women with COVID-19 had a fever, cough, shortness of breath, and sore throat. Clinical symptoms, common in severe acute respiratory infection, were also reported in other studies. Upper respiratory tract involvement was reported in various studies, indicating the transmission of infection to the upper respiratory tract (5, 12, 13). In the present study, 13 mothers had respiratory problems (76.5%), which is not consistent with the study of Fox and Melka in New York (14), in which people with two symptoms and positive PCR were included in the study out of 92 women with suspicious symptoms. It was found that 33 pregnant women were PCR-positive, 51 were not tested due to a lack of samples, eight women had a negative test while the family members were positive, which was probably a false negative, and only one person was hospitalized, and one woman was receiving oxygen at home. One person was admitted to the hospital again, which was also admitted in the 25th week of pregnancy, and one person was under oxygen therapy at home.
The early screening was done to identify mothers, but in the present study, hospitalized people were evaluated, and it seems that these people are more widely affected by the virus, or it may be due to the peak of COVID-19. Their study was done in the first peak for two months, but it was conducted for seven months in the present study. Pregnant women also had diarrhea and vomiting symptoms, indicating the virus's transmission to the gastrointestinal tract, consistent with the results of Chen et al., Song et al., Huang et al., Wang et al., Chen et al., and Chan et al. (12, 15-19). The subjects also had kidney and heart damage, consistent with studies by Tavakoli et al., and Liu et al. (20-22). Pregnant women are at risk of Coronavirus infection due to immunosuppression in pregnancy (13).
In the present study, the vertical transmission was observed in neonates. It is the transmission of a pathogen from mother to baby during the prenatal and postnatal periods. It is through the placenta during pregnancy, the birth canal, and lactation during postpartum. The present study findings are inconsistent with those of Liu et al. and Liu et al. (22, 23) and Chen et al. (10). These studies conducted in Wuhan, China, only enrolled mothers exposed to COVID-19 in the third trimester of pregnancy. In the study by Liu et al., only three mothers and infants were examined, and the difference can be due to the small sample size (23); also, Chen et al. investigated seven mothers, all in the third trimester of pregnancy (10). It seems that, COVID-19 vertical transmission like rubella virus is more likely to be transmitted during early pregnancy, but is less likely to be transmitted in the third trimester. Vertical transmission was not observed in the study by Liu et al. (23) in Wuhan, China, on pregnant women with > 35 weeks of gestational age. In the study by Liu et al. conducted in Wuhan, China, 19 pregnant mothers were examined. The mothers' ages were 27 - 34, and the gestational age in their study was 35 weeks or later. They did not have any underlying disease. Ten people with clinical symptoms and nine with positive PCR test results were reported. In their study, the vertical transmission was also not observed. The data show that the receptor of angiotensin-converting enzyme 2 has slight expression in the placenta, which makes vertical transmission impossible. According to the above information in this study, there were seemingly no cases of vertical transmission.
The present study results are consistent with Fenizia et al.'s (24) study in Italy on 31 mothers with COVID-19 that reported vertical transmission in a few subjects. In a study by Zhu et al. (13) in Wuhan, China, nine out of 10 infants were examined, and there was no vertical transmission. Placental tissue can be examined to better investigate the disease transmission pattern. More specific tests with IgG and IgM antibodies might be helpful in this regard. According to the research by Chan et al. (19) reporting that COVID-19 is more contagious than SARS, observing safety and health protocols is essential.
In the present study, pregnancy was terminated in 16 (94.1%) women with cesarean section, encompassing a large portion of deliveries. Based on Liu et al. (22) research in Wuhan, China, intensive care was required due to the complications of cesarean section.
In the present study, two infants were infected with COVID-19, consistent with Liu et al. (23) study in Wuhan, China. Infants' involvement in their study was 17 - 19 days after birth. In their study, contact with family members is mentioned as the cause of newborn conflicts; however, the disease incubation period is variable, and a false-negative test result is possible. The results of the study by Liu et al. were in line with those of Fenizia (24) conducted in Italy, indicating a more detailed examination and monitoring of infants in the NICU. He also reported the risk of disease transmission from the infant to medical staff, caregivers, and family members.
In the present study, 54.54% of infants were born prematurely, consistent with the study by Zhu et al. (13) in Wuhan, China. In their study, six out of 10 mothers examined gave birth to premature infants. In their study, six newborns were admitted due to critical problems. In the current study, nine newborns were admitted to the intensive care unit due to premature birth and one newborn due to a low Apgar score. It seems that COVID-19 infection may lead to hypoxia in women, which increases the risk of adverse perinatal complications, such as asphyxia at birth and preterm labor.
On the other hand, perinatal infection is a reason for giving birth to a preterm infant; the detailed examination of a fetus in terms of disease transmission might be helpful. For the better investigation of COVID-19 infection outcomes in infants, such as fetal distress, respiratory distress, and even death, it seems that the presence of neonatal specialists at delivery for taking immediate measures of infantile resuscitation, increasing survival rate, and improving infant prognosis is essential. Given the susceptibility of the mother and infant to the infection, strategies to prevent and control the disease might be helpful (25).
In the present study, 47% of women with COVID-19 were 20 - 30 years old. Considering the importance of pregnancy and maternal and child health and given the unknown complications of COVID-19, giving priority to young women's health is very important to prevent maternal complications until finding a definite remedy and vaccine against the virus.
In the present study, 11 (64.7%) mothers were admitted to the ICU. In a study by Fox and Melka (14) in New York, of 33 individuals with positive PCR results, only one needed hospitalization, and early identification seemed to reduce the hospital admission rate. The present study results are consistent with Breslin et al. (26). In their study, two out of seven individuals were admitted to the ICU. The present study findings are also consistent with those of Blitz et al. (27) in New York. They enrolled 70 pregnant women hospitalized due to COVID-19, of whom 13 (19%) were admitted to the ICU due to acute respiratory failure. In the present study, one (5.5%) woman died, consistent with the study by Blitz et al. (27) in New York that reported the death of two (15%) subjects; however, half of the patients had no underlying diseases.
5.1. Conclusions
Pregnant women may be more susceptible to respiratory infections and pneumonia than non-pregnant ones due to physiological changes in pregnancy, such as airway edema, diaphragm rise, increased oxygen consumption, and immune system alterations. Such changes make pregnant women less resistant to hypoxia and increase the risk of infection and its complications, such as preterm labor, infant infection, vertical transmission, and maternal morbidity and mortality. Given the new mutations in the virus and the unpredictability of its possible effects on mother and baby, it is recommended to inform mothers of possible outcomes before deciding to conceive. In addition, preparing ICUs and training staff are of great importance. Health workers are a vulnerable population group due to the risk of virus transmission. Midwifery care providers are at risk of occupational exposure to the virus due to long-time interactions with patients during childbirth and the unpredictable nature of midwifery care. The N95 filtering facepiece respirators are recommended for personnel exposed to definitely diagnosed or suspicious cases of COVID-19 until getting the test results. According to the obtained results, a meta-analysis might be helpful.
5.2. Limitations
Due to the limited PCR kits, vaginal and umbilical cord blood samples were not taken from all mothers. Specimens were not taken at pregnancy termination in case of bloody, meconium-stained, or low-quantity amniotic fluid. Due to the inappropriate condition of the mother, including fever and fetal tachycardia, sampling was omitted to reduce the incidence of unwanted complications. After recovery and discharge, two mothers terminated their pregnancies in another hospital and did not cooperate with the study. Due to the research team's involvement in the COVID-19 infection, the study progressed slowly.