1. Background
Infection with the novel coronavirus (COVID-19) has been shown to have significant deleterious effects on the cardiovascular system (1-5). The immune-mediated inflammatory response, endothelial dysfunction, and ensuing prothrombotic state can lead to micro and macrovascular thrombosis in these patients (1-16). The spectrum of macrovascular thrombosis includes venous thromboembolism, acute coronary syndrome, and stroke, with rare involvement of other visceral and peripheral arteries (1, 2, 4, 5, 8, 9, 11, 13, 14, 16). Thrombovascular complications have contributed significantly to morbidity and mortality, particularly in the elderly (2-4, 13, 15). Elevated D-dimer value in COVID-19 infection has been found to be associated with positive prediction of venous thromboembolism (1, 5, 8, 9, 11, 13-15, 17-20), increased risk of ischemic stroke (1, 5, 9, 16), acute myocardial injury, (1, 4, 5) and high risk of mortality (1, 6, 8, 10, 13-19). Guidelines throughout the world have recommended thromboprophylaxis for all hospitalized patients with COVID-19 infection (4, 7, 8, 10, 11, 13, 14, 18, 20, 21), with their D-dimer values as a guide (4, 10, 11, 13, 14, 20, 22, 23). Many of these have also recommended extending prophylactic anticoagulation for several weeks following discharge from the hospital (6-8, 11-13, 18, 20). This recommendation is largely based on the experience with sick hospitalized patients and the potential risk of persisting procoagulant state with new cardiovascular complications following recovery from acute COVID-19 (6, 10, 12-15, 20, 21). The D-dimer values and older age have been taken into consideration for recommending post-discharge prophylaxis in most of these guidelines (6, 10, 11, 13, 14, 18, 20, 22). The rationale is that elderly people and patients with elevated D-dimer values are the ones who are likely to get severe COVID-19 illness, with a high risk of vascular thrombosis, and hence are likely to benefit from extended prophylaxis. However, there are very limited data regarding the risk of developing macrovascular thrombosis following recovery from acute COVID-19 in the elderly, while the optimal duration of extended anticoagulation remains uncertain (6-8, 10-13, 18, 20).
2. Objectives
This study aimed at calculating the overall incidence and timing of macrovascular thrombosis in the elderly after 13 weeks of clinical recovery from acute COVID-19 infection and thereby assessing the need for and duration of extended thromboprophylaxis. It also aimed at finding out the predictive value of clinical severity, in-hospital anticoagulation, and discharge D-dimer values for the incidence of macrovascular thrombosis and overall mortality within 13 weeks following clinical recovery from acute COVID-19 infection in the elderly.
3. Methods
The data for the study were collected as part of a clinical trial (Clinical Trial Registry of India (CTRI) ref. no. CTRI/2020/11/029264) entitled “prevalence, pattern, and functional outcome of post-COVID-19 syndrome in older adults” done by the same authors for different objectives. The results of the trial were published earlier (24).
All elderly patients (at least 65-years-old) discharged between August 1, 2020, and November 30, 2020, with symptomatic acute COVID-19, were enrolled for the study at the time of discharge. In this study, COVID-19 was confirmed by real-time polymerase chain reaction (RT-PCR) from nasopharyngeal swabs. All the included patients were categorized clinically into mild-to-moderate (M), severe (S), and critical (C) illnesses based on the presentation and course of the illness in the hospital (25).
(1) Mild-to-moderate illness (M) included individuals who had any of the various signs and symptoms of COVID-19, with or without evidence of lower respiratory disease, but with oxygen saturation (SpO2) ≥ 94% in room air at the sea level.
(2) Severe illness (S) included individuals who had SpO2 < 94% in room air at the sea level.
(3) Critical illness (C) included individuals who had respiratory failure, septic shock, and/or multiple-organ dysfunction
The patients’ baseline characteristics including comorbidities were recorded from the medical records. The frequency of doing the D-dimer test and the use of prophylactic in-hospital anticoagulation were left at the treating physician’s discretion. When desired, either unfractionated heparin or low-molecular-weight heparin was used for anticoagulation. Most patients with normal D-dimer values on admission and with mild COVID-19 illness did not have a repeat test. Hence, only patients who had more than one D-dimer test done and had a D-dimer value done before discharge were included for the D-dimer group analysis and were divided into two groups, one with D-dimer values of less than or equal to two times the normal upper limit of the lab reference value and the other one with D-dimer values more than two times the normal upper limit of the lab reference value. Since the concept of extended thromboprophylaxis was evolving at that stage, none of the patients were put on anticoagulation during discharge.
All patients were contacted through telephone after 90 days (approximately 13 weeks) of discharge from the hospital, and details regarding the incidence of macrovascular thrombosis (acute coronary syndrome, acute stroke, venous thromboembolism, etc.) and death were collected through an interview. A maximum of five attempts over five days was made after the 90th day of discharge before declaring the patient as a non-responder. Macrovascular events were taken into account only if the patient or the attendee was able to provide the documented details of the event. Non-responders, due to various reasons (not responding to the call, changed numbers, not willing to participate, etc.), were excluded from the final analysis. Out of 335 discharges between August 1, 2020, and November 30, 2020, 47 patients were non-responders. Hence, the final analysis included 288 elderly patients.
Data were tabulated and analyzed with IBM SPSS Statistics for Windows, Version 23.0. (Armonk, NY: IBM Corp.). To describe the data, frequency analysis and percentage analysis were used for categorical variables, while mean and standard deviation (SD) were used for continuous variables. To find a significant difference between the bivariate samples in independent groups, the unpaired sample t test was used. For the multivariate analysis, the one-way ANOVA with Tukey's Post-Hoc test was used. To find the significance in categorical data, a chi-square test was used, and if the expected cell frequency was less than 5 in 2 × 2 tables, Fisher's Exact Test was used. In the above statistical tools, the probability value of 0.05 was considered the significance level. Cumulative incidence (CI) was calculated for macrovascular events at the end of 13 weeks for each group separately and expressed in percentage. Relative risk (RR) was calculated from the ratio between incidence rates of the two groups. The study was approved by the Institutional Ethics Committee-2.
4. Results
Out of 288 patients, 165 had mild to moderate (M) illness, 95 had severe (S) illness, and 28 had critical (C) illness (Table 1). The mean age of group C was significantly (P = 0.003) higher than that of groups M and S (74 years vs. 71 years and 72 years, respectively) (Table 1). The prevalence of all comorbidities other than diabetes mellitus was similar between the three groups (M, S, and C) (Table 1). Anticoagulation use was very high (> 95%) in the S and C groups than in the M group (P = 0.0005) (Table 1). The number of macrovascular thrombotic events was significantly higher in the C group than in the other two groups (17.9 vs. 1.8 and 1.1%, respectively) (P = 0.0005) (Table 1). Three (10.7%) patients died within 13 weeks of discharge in the C group versus one (0.6%) patient in the M group and none in the S group (P = 0.0005) (Table 1).
| Variables | Mild to Moderate (M); 165 (100) | Severe (S); 95 (100) | Critical (C); 28 (100) | P Value | Total; 288 (100) |
|---|---|---|---|---|---|
| Age | 71 ± 5 | 72 ± 6 | 74 ± 7 | 0.003 | |
| Male | 103 (62.4) | 61 (64.2) | 20 (71.4) | 0.655 | 184 (63.9) |
| Female | 62 (32.6) | 34 (35.8) | 8 (28.6) | 0.655 | 104 (36.1) |
| Diabetes mellitus | 82 (49.7) | 65 (68.4) | 15 (53.6) | 0.013 | 162 (56.3) |
| Hypertension | 104 (63) | 63 (66.3) | 16 (57.1) | 0.661 | 183 (63.5) |
| Coronary artery disease | 34 (20.6) | 20 (21.1) | 6 (21.4) | 0.993 | 60 (20.8) |
| Cerebrovascular disease | 4 (2.4) | 2 (2.1) | 2 (7.1) | 0.331 | 8 (2.8) |
| Chronic kidney disease | 6 (3.6) | 3 (3.2) | 3 (10.7) | 0.186 | 12 (4.2) |
| Hypothyroidism | 14 (8.5) | 9 (9.5) | 1 (3.6) | 0.607 | 24 (8.3) |
| Other comorbidities | 0.316 | ||||
| Chronic liver disease | 1 (0.60) | 0 (0) | 0 (0) | 1 (0.3) | |
| Obstructive airway disease | 7 (4.2) | 4 (4.2) | 1 (3.6) | 12 (4.1) | |
| Rheumatoid arthritis | 2 (1.2) | 1 (1) | 0 (0) | 3 (1.0) | |
| Peripheral neuropathy | 1 (0.60) | 0 (0) | 0 (0) | 1 (0.3) | |
| Psoriasis | 0 (0) | 1 (1) | 0 (0) | 1 (0.3) | |
| Seizure | 2 (1.2) | 2 (2.1) | 0 (0) | 4 (1.4) | |
| Parkinsonism | 0 (0) | 3 (3.1) | 2 (7.1) | 5 (1.7) | |
| Dementia | 0 (0) | 1 (1) | 1 (3.6) | 2 (0.7) | |
| Valvular heart disease | 2 (1.2) | 0 (0) | 0 (0) | 2 (0.7) | |
| Chronic atrial fibrillation | 0 (0) | 0 (0) | 1 (3.6) | 1 (0.3) | |
| Malignancies | 0 (0) | 2 (2.1) | 1 (3.6) | 3 (1.0) | |
| Sjogren’s disease | 1 (0.60) | 0 (0) | 0 (0) | 1 (0.3) | |
| Anticoagulation in hospital | 64 (38.8) | 94 (98.9) | 27 (96.4) | 0.0005 | 185 (64.2) |
| Number of macrovascular thrombotic events within 13 weeks of discharge | 3 (1.8) | 1 (1.1) | 5 (17.9) | 0.0005 | 9 (3.1) |
| Number of deaths within 13 weeks of discharge | 1 (0.6) | 0 (0) | 3 (10.7) | 0.0005 | 4 (1.4) |
a Values are expressed as No. (%) or mean ± SD.
Out of 288 elderly patients, 185 (64%) received anticoagulation in the hospital (Table 2). The mean age of the group that received anticoagulation in the hospital was higher (P = 0.028) than that of the group that did not receive (71.9 years vs. 70.5 years) (Table 2). The group that received anticoagulation included more severe (S) (50.8 vs. 1%) and critical (C) (14.6 vs. 1%) patients than the group that did not receive anticoagulation (P = 0.0005) (Table 2). The prevalence of comorbidities was similar between the two groups. Also, the numbers of macrovascular events [5 (2.7%) vs. 4 (3.9%)] and deaths [(3 (1.6%) vs. 1 (1%)] were not significantly different between the two groups (P = 0.726 and 1.000, respectively) (Table 2).
| Variables | Anticoagulation in Hospital/Yes; 185 (100) | Anticoagulation in Hospital/No; 103 (100) | P Value | Total; 288 (100) |
|---|---|---|---|---|
| Age (y) | 71.9 ± 6 | 70.5 ± 4.5 | 0.028 | |
| Male | 120 (64.9) | 64 (62.1) | 0.644 | 184 (63.9) |
| Female | 65 (35.1) | 39 (37.9) | 0.644 | 104 (36.1) |
| Mild/moderate | 64 (34.6) | 101 (98.1) | 0.0005 | 165 (57.3) |
| Severe | 94 (50.8) | 1 (1) | 0.0005 | 95 (33) |
| Critical | 27 (14.6) | 1 (1) | 0.0005 | 28 (9.7) |
| Diabetes mellitus | 115 (62.2) | 47 (45.6) | 0.007 | 162 (56.3) |
| Hypertension | 120 (64.9) | 63 (61.2) | 0.632 | 183 (63.5) |
| Coronary artery disease | 38 (20.5) | 22 (21.4) | 0.870 | 60 (20.8) |
| Cerebrovascular disease | 5 (2.7) | 3 (2.9) | 1.000 | 8 (2.8) |
| Chronic kidney disease | 9 (4.9) | 3 (2.9) | 0.547 | 12 (4.2) |
| Hypothyroidism | 16 (8.6) | 8 (7.8) | 0.795 | 24 (8.3) |
| Other comorbidities | 0.290 | |||
| Obstructive airway disease | 9 (4.9) | 3 (2.9) | 12 (4.1) | |
| Rheumatoid arthritis | 1 (0.5) | 2 (1.9) | 3 (1) | |
| Peripheral neuropathy | 0 (0) | 1 (1) | 1 (0.3) | |
| Psoriasis | 1 (0.5) | 0 (0) | 1 (0.3) | |
| Seizure | 3 (1.6) | 1 (1) | 4 (1.4) | |
| Parkinsonism | 5 | 0 (0) | 5 (1.7) | |
| Dementia | 2 | 0 (0) | 2 (0.7) | |
| Valvular heart disease | 1 (0.5) | 1 (1) | 2 (0.7) | |
| Chronic atrial fibrillation | 1 (0.5) | 0 (0) | 1 (0.3) | |
| Malignancies | 3 (1.6) | 0 (0) | 3 (1) | |
| Sjogren’s disease | 0 (0) | 1 (1) | 1 (0.3) | |
| Number of macrovascular thrombotic events within 13 weeks of discharge | 5 (2.7) | 4 (3.9) | 0.726 | 9 (3.1) |
| Number of deaths within 13 weeks of discharge | 3 (1.6) | 1 (1) | 1.000 | 4 (1.4) |
a Values are expressed as No. (%) or mean ± SD.
Discharge D-dimer values were available for 103 patients and were used for the analysis (Table 3). The patients who had elevated D-dimer values (> 2 times the normal upper limit) at discharge, despite a clinical recovery, were older (median age 74.8 years vs. 70.7 years) than patients with reduced D-dimer values (≤ 2 times the normal upper limit) at discharge (P = 0.026) (Table 3). The prevalence of critical illness was more (33.3 vs. 10.1%; P = 0.022) in the group with elevated D-dimer values at discharge than in the group with reduced D-dimer values at discharge (Table 3). There were two macrovascular thrombotic events at the end of the 13 weeks follow-up period in the elevated D-dimer group, versus one event in the reduced D-dimer group (P = 0.135). The number of deaths was higher in the elevated D-dimer group [2 (8.3%) vs. 0 (0)] but did not achieve statistical significance (P = 0.053) (Table 3).
| Variables | Reduced D-Dimer Values at Discharge (≤ 2 Times the Normal Upper Limit); 79 (100) | Elevated D-Dimer Values at Discharge (> 2 Times the Normal Upper Limit); 24 (100) | P Value | Total; 103 (100) |
|---|---|---|---|---|
| Age | 70.7 ± 5.5 | 74.8 ± 7.9 | 0.026 | |
| Male | 47 (59.5) | 21 (87.5) | 0.013 | 68 (66) |
| Female | 32 (40.5) | 3 (12.5) | 0.013 | 35 (34) |
| Mild/moderate | 41 (51.9) | 10 (41.7) | 0.022 | 51 (49.5) |
| Severe | 30 (38) | 6 (25) | 0.022 | 36 (35) |
| Critical | 8 (10.1) | 8 (33.3) | 0.022 | 16 (15.5) |
| Diabetes mellitus | 47 (59.5) | 11 (45.8) | 0.237 | 58 (56.3) |
| Hypertension | 50 (63.3) | 12 (50) | 0.244 | 62 (60.2) |
| Coronary artery disease | 13 (16.5) | 5 (20.8) | 0.621 | 18 (17.5) |
| Cerebrovascular disease | 4 (5.1) | 0 (0) | 0.571 | 4 (3.9) |
| Chronic kidney disease | 2 (2.5) | 2 (8.3) | 0.231 | 4 (3.9) |
| Hypothyroidism | 4 (5.1) | 0 (0) | 0.571 | 4 (3.9) |
| Other comorbidities | 0.514 | |||
| Chronic liver disease | 1 (1.3) | 0 (0) | 1 (1) | |
| Obstructive airway disease | 4 (5.1) | 0 (0) | 4 (3.9) | |
| Rheumatoid arthritis | 0 (0) | 0 (0) | 0 (0) | |
| Peripheral neuropathy | 0 (0) | 0 (0) | 0 (0) | |
| Psoriasis | 1 (1.3) | 0 (0) | 1 (1) | |
| Seizure | 1 (1.3) | 0 (0) | 1 (1) | |
| Parkinsonism | 1 (1.3) | 2 (8.3) | 3 (2.9) | |
| Dementia | 1 (1.3) | 1 (4.1) | 2 (1.9) | |
| Valvular heart disease | 0 (0) | 0 (0) | 0 (0) | |
| Chronic atrial fibrillation | 0 (0) | 0 (0) | 0 (0) | |
| Malignancies | 2 (2.5) | 0 (0) | 2 (1.9) | |
| Sjogren’s disease | 0 (0) | 0 (0) | 0 (0) | |
| Anticoagulation in hospital | 64 (81) | 23 (95.8) | 0.109 | 87 (84.5) |
| Number of macrovascular thrombotic events within 13 weeks of discharge | 1 (1.3) | 2 (8.3) | 0.135 | 3 (2.9) |
| Number of deaths within 13 weeks of discharge | 0 (0) | 2 (8.3) | 0.053 | 2 (1.9) |
a Values are expressed as No. (%) or mean ± SD.
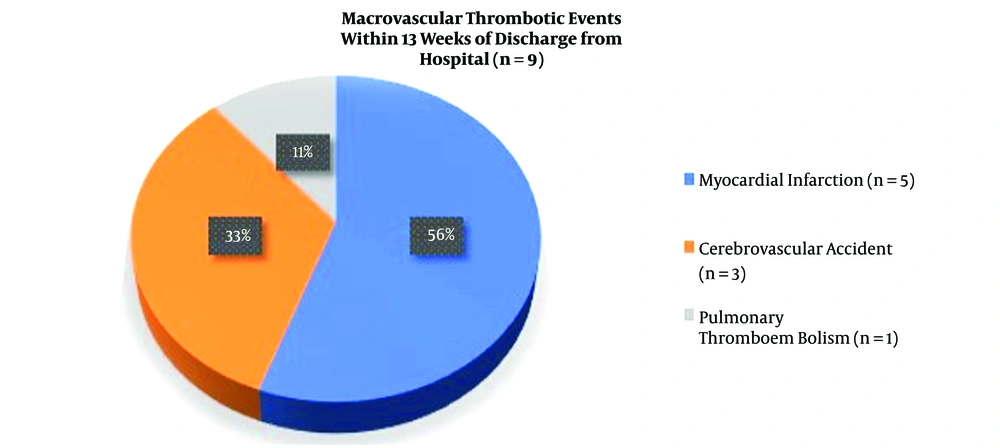
There were nine macrovascular thrombotic events within 13 weeks of discharge from the hospital among the followed-up elderly. The most common event was acute myocardial infarction (56%), followed by acute cerebrovascular accident (33%) and pulmonary thromboembolism (11%) (Figure 1).
In the initial four weeks from the date of discharge, one macrovascular thrombotic event occurred while there were no deaths (Table 4). The majority of the macrovascular thrombotic events (six out of nine) occurred between the fourth week and the eighth week of discharge from the hospital (Table 4). Similarly, two out of three deaths occurred between the fourth week and the eighth week of discharge in the followed-up elderly population (Table 4). After the eighth week of discharge till the end of the follow-up period (13 weeks), two macrovascular thrombotic events occurred out of which one patient died. During the same period, one patient died of other causes (sepsis) (Table 4).
| Duration of Discharge from the Hospital | Number of Macrovascular Thrombotic Events | Number of Macrovascular Thrombosis-Related Deaths | Number of Deaths Due to Other Causes (Sepsis) |
|---|---|---|---|
| 0 - 4 weeks | 1 | 0 | 0 |
| 4 - 8 weeks | 6 | 2 | 0 |
| 8 - 13 weeks | 2 | 1 | 1 |
| Total | 9 | 3 | 1 |
The cumulative incidence rate of macrovascular events in the post-COVID-19 elderly cohort at 13 weeks after discharge was 3.12% (Table 5). The incidence rate was the highest for the critical illness group (17.85%) and the lowest for the severe illness group (1.05%) (Table 5). In comparison with the whole post-COVID-19 elderly cohort, the relative risk was very high for the critically ill elderly (5.77 vs. 1) and the elderly with elevated D-dimer values at discharge (2.67 vs. 1) (Table 5). Elderly patients who did not receive anticoagulation in the hospital had a relative risk of 1.24 (Table 5). The relative risk was lower for elderly patients with severe illness (0.34), mild to moderate illness (0.58), reduced D-dimer values at discharge (0.40), and those who received anticoagulation in hospital (0.86) (Table 5).
| Study Cohort | Numbers Followed Up Till 13 Weeks | Number of Macrovascular Thrombotic Events | Cumulative Risk at 13 Weeks | Relative Risk |
|---|---|---|---|---|
| Post-COVID-19 elderly | 288 | 9 | 3.12 | 1 |
| Mild to moderate (M) | 165 | 3 | 1.82 | 0.58 |
| Severe (S) | 95 | 1 | 1.05 | 0.34 |
| Critical (C) | 28 | 5 | 17.85 | 5.72 |
| Receiving anticoagulation in hospital/Yes | 185 | 5 | 2.7 | 0.86 |
| Receiving anticoagulation in hospital/No | 103 | 4 | 3.88 | 1.24 |
| Reduced D-dimer values at discharge (≤ 2 times the normal upper limit) | 79 | 1 | 1.26 | 0.40 |
| Elevated D-dimer values at discharge (> 2 times the normal upper limit); 24 (100) | 24 | 2 | 8.33 | 2.67 |
5. Discussion
Our study included 288 elderly patients, the majority of whom (63.9%) were males (Table 1). Most of the earlier studies have shown similar gender distribution among the hospitalized COVID-19 patients (1, 2, 4, 26). Increasing age has been shown to be associated with increased hospitalization, severe clinical illness, and a high mortality rate due to COVID-19 infection (1-4, 6, 11, 13, 15, 18, 20, 27). Our study also showed that the mean age increased significantly as the clinical severity increased, with the critical illness (C) group consisting of older patients than the severe illness (S) and mild to moderate illness (M) groups (mean age 74 years vs. 72 years and 71 years, respectively) (Table 1). There seems to be a linear correlation between age and the severity of clinical illness in COVID-19 infection. The use of anticoagulation was very higher in the severe illness (S) group and critical illness (C) group than in the mild to moderate (M) group (Table 1). Even though the recent consensus is to use anticoagulation for all hospitalized patients, some of the earlier guidelines had suggested the use of the same in COVID-19 patients with severe clinical illness and low bleeding risk (6, 7, 11, 13, 14, 20-22, 25, 27, 28). This might be the reason for the less use of anticoagulation in the M group. During the follow-up period of 13 weeks, the patients with critical clinical illness during hospitalization had significantly higher numbers of macrovascular events (17%) and deaths (10.7%) than the mild to moderate and severe illness groups (P = 0.0005) (Table 1). It is reasonable to say that critically ill elderly patients have a higher prevalence of macrovascular thrombosis and death in the post-COVID-19 period than the elderly with mild to severe clinical illness due to COVID-19.
Anticoagulation with heparin in hospitalized COVID-19 patients has been shown to bring down the in-hospital thrombotic risk and mortality in all patients (5, 7, 10, 11, 13, 14, 17-19, 26-28) as well as the 28-day mortality and thrombotic risk in critically ill patients (7, 13, 14). Accordingly, current guidelines recommend anticoagulation for all hospitalized patients. Whether in-hospital anticoagulation will reduce the risk of thrombosis and mortality in the post-COVID-19 period has not been studied so far. We followed up two elderly cohorts, with and without in-hospital heparin use, up to 13 weeks for determining the same (Table 2). The cohorts differed in age, with patients receiving in-hospital anticoagulation being significantly older (mean age 71.9 years vs. 70.5 years) and predominantly consisting of severe and critically ill patients (P = 0.0005) (Table 2). Despite these differences, the prevalence of thrombotic events and deaths at the end of 13 weeks did not differ significantly between the two groups (Table 2). Hence, in-hospital anticoagulation does not seem to provide significant protection against long-term macrovascular thrombotic risk and mortality in the post-COVID-19 elderly.
Extended thromboprophylaxis for post-COVID-19 patients has been recommended by several guidelines for high-risk groups, as the risk of thrombosis might extend beyond clinical recovery in them (6, 7, 10-14, 18-22, 27, 28). The two common factors that are considered for deciding on the risk of thrombosis are advanced age and D-dimer values > 2 times the upper limit of normal value (6, 13, 14, 18, 20-22, 27, 28). These recommendations were based on D-dimer values on admission or D-dimer values during the course of illness. We thought that D-dimer values at discharge might be a better measure to estimate the prothrombotic risk in the post-COVID-19 period and hence, enrolled patients with discharge D-dimer values in our study (Table 3). Our analysis showed that the mean age of the abnormal D-dimer group (> 2 times the discharge D-dimer value after clinical recovery) was significantly higher (74.8 years vs. 70.7 years) and had more numbers of critically ill patients (33.3 vs. 10%). The prevalence rate of macrovascular thrombosis was higher in the abnormal D-dimer group (8.3 vs. 1.3%) but was not statistically significant. However, there were two deaths in the abnormal D-dimer group while there were none in the normal D-dimer group (P = 0.053). Increased D-dimer levels at discharge, more than two times the normal value, might be a better risk factor for assessing the need for thromboprophylaxis in the post-COVID-19 elderly.
The most common macrovascular thrombotic event observed in our study population at the end of the follow-up period was acute myocardial infarction (56%), followed by cerebrovascular accident (33%) and pulmonary thromboembolism (11%) (Figure 1). Though this pattern is similar to macrovascular complications reported earlier by several authors in acute COVID-19 infection (5, 9, 14, 16, 17, 21, 25-27, 29), the prevalence of myocardial infarction was more than that of venous thromboembolism (Figure 1). In the 30 days’ post-COVID-19 follow-up study by Patell et al., there were one event of stroke and one event of pulmonary thromboembolism (12). Our analysis suggests that the risk of arterial thrombosis is more than that of venous thrombosis in the post-COVID-19 period. Nevertheless, more comprehensive studies are needed to confirm this.
The cumulative incidence of macrovascular thrombosis in the hospitalized post-COVID-19 elderly was 3.12% at 13 weeks post-discharge (Table 4). Patell et al. also reported a cumulative incidence of 2.5% in post-COVID-19 patients with a median follow-up period of 30 days (12). The higher incidence in our study was likely to be because of the older study population and longer follow-up period. The cumulative incidence and relative risk were the highest for the critical illness group (17.85 and 5.72%, respectively), followed by the abnormal D-dimer group (8.33 and 2.67%, respectively) (Table 4). Critical illness and elevated D-dimer values at discharge seem to pose a higher risk of macrovascular thrombosis in the post-COVID-19 elderly. However, for unclear reasons, the incidence rate was lower in the severe group than in the mild to moderate group (1.04 vs. 1.82%) (Table 4). The reasons might be the lower follow-up sample size (95 vs. 165) and other risk factors that were not taken into account, such as obesity, dyslipidemia, etc.
The timing of the incidence of macrovascular thrombotic events might guide us in deciding on the duration of extended thrombotic prophylaxis in the post-COVID-19 elderly, which has been unclear till now. In our study, the maximum number of macrovascular thrombotic events (six out of nine, 78%) and related deaths (two out of three, 67%) occurred before eight weeks from the date of discharge from the hospital (Table 5). Hence, it is reasonable to conclude that the minimum duration of extended thromboprophylaxis in the post-COVID-19 elderly should be at least eight weeks to achieve the maximum benefits from it.
5.1. Strengths and Limitations
To the best of our knowledge, this is the first follow-up study done exclusively on the elderly cohort population to assess the risk of macrovascular thrombosis following clinical recovery from COVID-19 infection. The non-responders during the follow-up were less than 15% of the whole study population.
Having said all of this, there are many limitations to our study. There is no normal cohort being followed up for comparison. The mean duration of in-hospital anticoagulation was not taken into account, as it was left to the physician’s discretion. Information about the thrombotic events was collected through the telephone even though all possible efforts were done to verify the documents. There is a possibility of the influence of risk factors such as antiplatelet agent use, dyslipidemia, and obesity, which were not considered in the analysis.
5.2. Conclusions
The cumulative incidence of macrovascular thrombotic events in the post-COVID-19 elderly was about 3.12%. The most common macrovascular event was myocardial infarction, followed by a cerebrovascular accident. In-hospital anticoagulation offered marginal protection for macrovascular thrombosis in the post-COVID-19 elderly. Elderly patients with a critical illness during hospitalization due to COVID-19, along with ones with discharge D-dimer values more than two times the normal limit, had the maximum risk of developing macrovascular thrombosis within 13 weeks of discharge from the hospital after clinical recovery. It is reasonable to recommend extended thromboprophylaxis for at least eight weeks in the post-COVID-19 elderly for achieving maximum benefits. However, considering the limitations of this study, more comprehensive controlled trials are needed to assess the risk of macrovascular thrombosis and the need for extended prophylaxis in the elderly, considering their high bleeding risk.