1. Background
Coronaviruses are a large group of viruses that cause diseases in both humans and animals. They cause human respiratory infections of varying severity levels ranging from mild to severe (1, 2). In late 2019, a new coronavirus, called severe acute respiratory syndrome coronavirus 2, caused an outbreak of unusual viral pneumonia in humans (3, 4). This highly contagious coronavirus was transmitted from an animal to a human for the first time, leading to the person-to-person transmission of the disease. In the beginning, the virus spread increasingly in Wuhan, China, and then it spread rapidly worldwide (5). It is currently estimated that 80,989,513 individuals in 24 countries have been infected by the disease, and more than 1,769,855 individuals have died from the disease. In addition, these statistics are still increasing day by day (6, 7). Patients with the severe form of the disease account for approximately 15% of coronavirus disease 2019 (COVID-19) cases; therefore, the coronavirus pandemic is considered a public health emergency and a global concern (8).
Since the onset of the COVID-19 pandemic, several treatment regimens have been prescribed; however, the efficacy of none of them has been proven, and worldwide efforts to find effective treatments are still in progress (9, 10). However, antiviral therapy seems to be necessary for epidemic diseases. Therefore, various studies are currently pending to evaluate the effectiveness of these drugs since the conclusive recommendation for many of these treatment regimens is still not available. For this reason, since the beginning of the epidemic, several types of drugs, such as antivirals, antimalarial medications, favipiravir, remdesivir, corticosteroids, immunoglobulin, cytokine, lopinavir/ritonavir, and hydroxychloroquine, have been investigated and suggested as adjunctive therapies for COVID-19 treatment. The results of these treatments are either inconclusive or inconsistent across studies (11-14). Interferon (INF) compounds are among the drugs whose effectiveness is still controversial (15, 16).
The IFNs are proteins belonging to the cytokine group. Type I IFNs, including IFN-α and IFN-β, are the most important IFNs. These compounds can have a direct inhibitory effect on the replication of some viruses and stimulate the immune system (17). The INF antiviral activity has already been investigated in various studies, and some studies in animal models have presented promising results (18). For instance, INF-β-1 has demonstrated the strong inhibition of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus in laboratory studies (12); nevertheless, other studies reported some contradictory results (19-22).
2. Objectives
The present study aimed to compare the safety and efficacy of a treatment regimen containing INF-β-1b with a standard INF-free treatment regimen in patients with severe COVID-19 through a randomized controlled trial study.
3. Methods
3.1. Setting
In this open-label, randomized controlled trial, severe cases of COVID-19 in Dr. Ganjavian hospital in Dezful, Iran, were included. This hospital is one of the major referral centers for COVID-19 in the southwest of Iran.
3.2. Patients
The participants of this research were the patients that (1) were aged 18 or over; (2) had either radiological symptoms or a positive polymerase chain reaction (PCR) result affirming the diagnosis of COVID-19; and (3) were in the severe stage of the disease.
Following the National Institutes of Health guideline, the patients were considered to be in the severe stage of the disease with peripheral oxygen saturation (SpO2) < 94% on room air and at sea level, a ratio of partial pressure of oxygen/fraction of inspired oxygen < 300 mm Hg, and respiratory rate > 30 breaths per minute or lung infiltrates > 50% (15). The patients were excluded from the study if they met one or more of the following criteria:
- They were unwilling to participate in the study.
- They manifested severe drug side effects.
- They had a history of allergy to similar compounds.
- They were pregnant or breastfeeding.
- They had a life expectancy of fewer than 48 hours due to the severity of the disease.
3.3. Informed Consent
The present study was carried out according to the Helsinki Declaration. Informed consent documents were obtained from the patients for participating in this study. The treatment protocol in this study was approved and registered with a code number by the Ethics Committee of Dezful University of Medical Sciences (IR.DUMS.REC.1399.031) and the Iranian Registry of Clinical Trials (IRCT20200921048786N1). The onset, progress, and flow of the study were evaluated and supervised by a three-member monitoring team during the study. The team was also responsible for registering and monitoring the data collected during the study.
3.4. Interventions
The patients were divided into two groups by block randomization. The intervention group was assigned to receive the standard regimen and five to seven 250-mcg doses of IFN-β-1b (Interferon β-1b®, Darou Pakhsh Co., Iran), which were administrated subcutaneously every other day. Both groups, including the control group, were treated with the national standard regimen of Kaletra (lopinavir/ritonavir; 400/100 mg every 12 hours) and hydroxychloroquine (400 mg every 12 hours on the first day and then 200 mg every 12 hours) for 7 - 10 days. The aforementioned treatment regimens were recommended by the Iranian National Guideline and some foreign guidelines for patients with COVID-19 when this study was initiated (23-25).
The patients of both groups were followed up daily for the response to treatment and side effects until the end of the study. Due to the possible effect of the combination of hydroxychloroquine and Kaletra on the QT interval, all patients were monitored daily for electrocardiogram changes at the beginning of hospitalization and then during the study. In cases where the QT interval was above 440 milliseconds, the dose of the causative drug was reduced. Moreover, in cases where the QT interval was above 500 milliseconds, the relevant treatment was discontinued. Additionally, for all the patients in the intervention group, the complete blood count with differential was checked every other day due to the possible hematological complications caused by INF β-1b, and the drug dose was reduced for the patients that got leukopenia or manifested thrombocytopenia after the intervention. To control fever and local complications of INF injection, the patients in the intervention group were given a single dose of 500 mg naproxen 30 minutes before the INF injection.
3.5. Outcomes
The primary outcome of this study was the evaluation of recovery and mortality rates in both groups. The criteria for patients’ recovery included the absence of respiratory distress, SpO2 > 93% without using supplemental oxygen, normal body temperature, and reduction or absence of cough at the end of the study. The secondary outcome was to compare intubation cases in the two groups. Instead of intensive care unit (ICU) admissions, intubation cases were considered the aim of the study due to the limited number of ICU beds in this therapeutic center, the large number of patients in need of ICU hospitalization, and avoiding possible bias errors during data analysis. Additionally, lactate dehydrogenase (LDH) and oxygen (O2) saturation levels were considered to be two predictive factors of disease severity. The measurements of LDH and O2 saturation levels at the beginning of hospitalization and later at the end of the study were used to calculate and compare the average levels for the two groups. Another purpose of this study was to investigate the average length of hospital stay in both groups.
3.6. Sampling Method
3.6.1. Randomization and Blinding
The sample size was calculated based on the sample size equation for the comparison of two means that obtained 90 patients (45 per group) with 80% power (β = 0.20) and α = 0.05. In the equation, the effect size was the mean length of hospital stay in the intervention and control groups reported as 11 ± 1.23 and 13 ± 1.58 days (14).
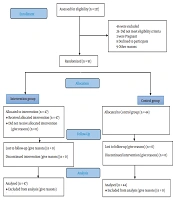
A total of 91 patients were eligible for this study based on the criteria explained in section 3.2. These patients were divided almost equally into two intervention and control groups. The random assignment of the patients to these two groups was carried out using a blocked randomization method. A block size of 4 was used in this study. Random block selection required the random assignment of two patients to the intervention (I) and the other two to the control (C) groups. There were only six possible blocks for this problem, including IICC, ICIC, ICCI, CCII, CICI, and CIIC, which were numbered 1 to 6. One of these blocks was selected using simple random sampling. For a population of 91 patients, 22 blocks (a total of 88 patients) were needed. Therefore, the process of random block selection was conducted 22 times. All the 22 subsequences (e.g., IICC and ICIC) generated by random block selection were put together to form a larger sequence, and then a number from 1 to 88 was assigned to each of its letters. The remaining three patients were assigned to the intervention group. Ultimately, 47 patients were randomly assigned to the intervention group and 44 patients to the control group. All the researchers except the main researcher were unaware of the block size and the generated block sequence. The data required for the study was first collected and recorded on paper forms during the study.
3.7. Statistical Analysis
The collected data were reviewed and monitored by a three-member team during the study. The data were analyzed using SPSS software (version 24.0). A P-value of less than 0.05 was considered the significance level. Descriptive statistics (e.g., percentage, mean, and standard deviation) were used for patients’ demographic data. The chi-square test was used to compare the mortality and intubation rates between the two groups. In addition, the t-test was used to compare the length of hospital stay, the percentage of blood O2 saturation, and LDH level (measured at the beginning and the end of the study) between the two groups (Figure 1). The sample size was calculated by the following equation:
4. Results
Out of the 137 patients who were screened according to the inclusion criteria, 91 patients [including 59 males (64.8%) and 32 females (35.2%)] were eligible and therefore included in the study. In this study, 44 and 47 patients were assigned to the control and intervention groups using the blocked randomization method, respectively. The mean age of the participants was 38 years (38 ± 15.65). Moreover, 50 patients had no history of diseases; however, 41 patients had a history of at least one underlying disease. The most common underlying diseases, ordered according to the prevalence, included diabetes (n = 32; 32.35%), hypertension (n = 21; 23.07%), chronic kidney disease (n = 3; 3.30%), and acute kidney injury (n = 1; 1.10%) (Table 1).
| Variables and Groups | Intervention Group (n = 47) | Control Group (n = 44) | Total (n = 91) | P-Value b |
|---|---|---|---|---|
| Age | 55.57 (32 - 98) | 63.45 (27 - 88) | 59.38 (27 - 98) | 0.05 |
| Gender | 0.43 | |||
| Male | 31 (65.96) | 28 (63.64) | 59 (64.83) | |
| Female | 16 (34.04) | 16 (36.36) | 38 (35.17) | |
| Comorbidity | 0.18 | |||
| None | 26 (55.32) | 24 (54.54) | 50 (54.94) | |
| DM | 18 (38.30) | 14 (31.81) | 32 (35.16) | |
| Hypertension | 9 (19.15) | 12 (27.27) | 21 (23.08) | |
| CKD | 3 (6.38) | 0 (0) | 3 (3.30) | |
| AKI | 1 (2.12) | 0 (0) | 1 (1.10) | |
| LDH level on admission IU | 717.38 ± 264.39 | 603.70 ± 193.34 | 0.01 | |
| SpO2 on admission (%) | 87.32 ± 4.93 | 88.59 ± 8.17 | 0.18 |
Abbreviations: SD, standard deviation; DM, diabetes mellitus; CKD, chronic kidney disease; AKI, acute kidney injury; LDH, lactate dehydrogenase; SpO2, peripheral oxygen saturation.
a Values are expressed as mean ± SD or No. (%).
b Significance level: P < 0.05
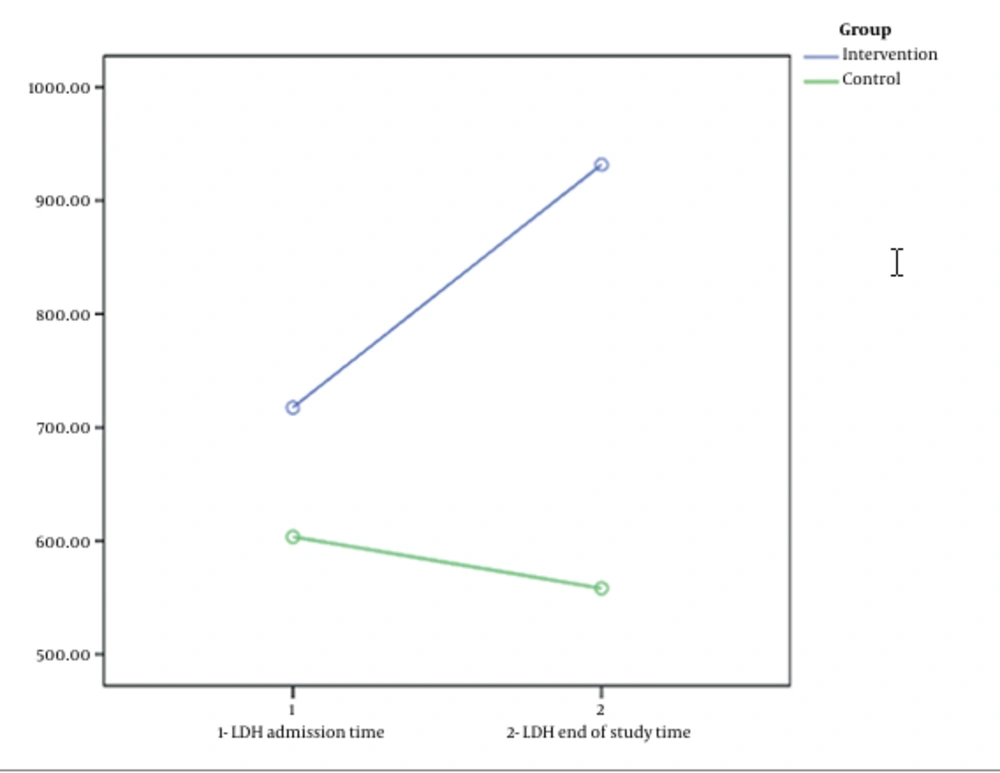
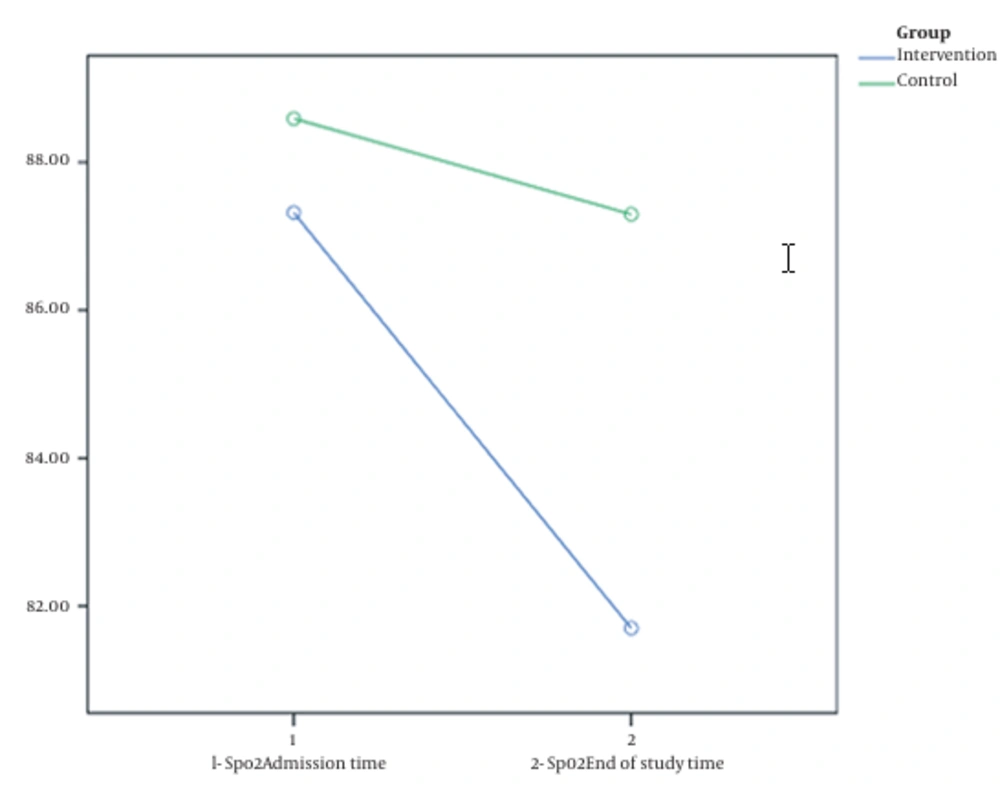
The length of hospital stay in the intervention group was significantly longer than in the control group (13.21 ± 6.88 vs. 10.52 ± 5.77 days; P = 0.047). The mortality rate did not differ significantly between the intervention and control groups (19.15% and 13.64%, respectively; P = 0.509). The intubation rate did not differ significantly between the intervention and control groups (12.76% and 11.36%, respectively; P = 0.838). There was no significant difference between the mean LDH and the mean SpO2 before and after the intervention in either group (P > 0.05). Nevertheless, on the last day of hospitalization, the mean O2 saturation of the intervention group was lower than the control group (81.7 ± 22.28 vs. 87.29 ± 17.30), which significantly differed (P = 0.01). In addition, at the end of the study, the LDH level of the intervention group was higher than the control group (949.20 ± 230.51 vs. 543.31 ± 193.33), which significantly differed (P = 0.01) (Table 2).
| Study Groups | Intervention Group | Control Group | P-Value* | Total |
|---|---|---|---|---|
| Length of hospital stay (day) | 13.21 ± 6.88 | 10.52 ± 5.77 | 0.047 | 11.91 ± 6.48 |
| Mortality rate | 9 (19.15) | 6 (13.64) | 0.509 | 15 (16.48) |
| Intubation rate | 6 (12.76) | 5 (11.36) | 0.838 | 11 (12.09) |
| Final SpO2 (%); median ± SD | 81.7 ± 22.28 | 87.29 ± 17.30 | 0.01 | - |
| Final LDH (%) | 949.20 ± 230.51 | 543.31 ± 193.33 | 0.01 | - |
Abbreviations: SD, standard deviation; SpO2, peripheral oxygen saturation; LDH, lactate dehydrogenase.
a Values are expressed as mean ± SD or No. (%) unless otherwise indicated.
b Significance level: P < 0.05
5. Discussion
This study aimed to compare the safety and efficacy of the INF-β-b1 treatment regimen with a standard INF-free treatment regimen in patients with severe COVID-19 through a clinical trial. The results of the present study showed that taking INF-β-1b did not lead to the recovery of patients with a severe form of COVID-19 in comparison to the patients who did not receive INF-β-1b (9 vs. 6 deaths). The number of recovered (discharged) cases was the same (n = 38) for both groups. Additionally, the intubation rate of the patients with severe COVID-19 was not significantly different for the group receiving INF-β-1b and the control group (6 vs. 5 cases).
Ethically, during the course of this study, the researchers had to ensure that the patients were receiving a proper treatment regimen and were not deprived of the necessary medication. Therefore, hydroxychloroquine and Kaletra were prescribed for all the patients according to the therapeutic recommendations of the Iranian National Guideline for COVID-19, which are similar to the recommendations provided by some international guidelines (26, 27). With regard to the present study, a study conducted by Al-Tawfiq et al. evaluated the effects of ribavirin and IFN combination in patients with the Middle East respiratory syndrome. They used two doses of IFN-α-2b (100 μg) subcutaneously once a week. They reported that ribavirin and IFN could be effective in some patients but were associated with mortality in severe patients. Moreover, they found that the compound was more helpful in patients who were not severely ill (19).
A study by Estébanez et al. also stated that the treatment with INF-β-1b was not significantly associated with the reduction of in-hospital mortality in patients hospitalized with COVID-19 (12).
However, this result contradicts the results of a study by Dastan et al., which confirmed the use of IFN-β-1a in combination with hydroxychloroquine and lopinavir/ritonavir for the management of COVID-19 (26). This difference in their results might be due to the lack of a control group, the effects of concomitant drug use, and the small sample size.
Other findings of this study regarding serum LDH and SpO2 levels seemed to be important, although they were not statistically significant. The mean serum LDH levels of the two groups were close on admission time (the difference was about 100 units); nevertheless, after the intervention, this difference increased a great deal. At the end of the study, the mean serum LDH level in the control group decreased to 543 U/L; however, in the intervention group, it significantly increased to 949 U/L (Figure 1). Similarly, the results of other studies (15, 27) have shown that serum LDH and qualitative C-reactive protein (CRP) are potentially helpful follow-up parameters in COVID-19 pneumonia, which help diagnose disease progression, and early intervention. Since it was impossible to quantitatively measure CRP in this study, it was tried to accurately record the numerical values of serum LDH at the beginning of hospitalization before the intervention and after the intervention at the end of the study. Accordingly, more patients in the intervention group entered more severe stages of the disease after receiving INF-β-1b, although the judgment was not exclusively based on a laboratory test.
The second similar finding was about the SpO2 parameter. The mean value of SpO2 at the beginning of hospitalization was almost the same (with a difference of about 1%) for both groups. Although after the intervention and receiving the respective treatment regimens, an unexpected difference was observed in the average SpO2 of the two groups; accordingly, at the end of the study, it averaged 81.7% in the intervention group versus 87.29% in the control group (Figure 3).
The results of this study showed that the administration of INF-β-1b in severe cases of COVID-19 might not only not help improve patients’ clinical condition but also exacerbate the disease and make it more difficult for the physician to control it in some cases. This could also be the cause for the prolongation of the hospital stay for the patients in the intervention group), as according to the findings, the duration of hospitalization was significantly different (P = 0.047) between the two groups; the mean values of hospitalization length were 13.21 and 10.52 days in the intervention and control groups, respectively. It is true that INF compounds can have antiviral effects (17.18); however, the researchers believe that the administration of this drug in severe cases of the disease by stimulating the immune system and disrupting the balance between inflammatory and anti-inflammatory cytokines aggravates the patient’s clinical conditions. Therefore, in this condition, more time and therapeutic measures are necessary to control the inflammatory process.
At the time of this study, there was insufficient evidence of INF efficacy in COVID-19. However, sometime later, the results of studies showed that INF compounds do not help control the disease, and even in severe cases, they can be harmful, similar to what was obtained in the current study (15, 27).
The findings of this study regarding the indication for INF taking in advanced and severe cases of COVID-19 are in accordance with the latest recommendations of authoritative scientific sources (16). Considering the physiopathology of COVID-19 and the functional mechanism of INF-β-1b, it seems that the administration of INF compounds is not helpful in severe cases of the disease. The results of a study by Ranieri et al. showed that IFN- β-1a is ineffective in the treatment of acute respiratory distress syndrome and is not a proper therapeutic agent for patients at the critical stage (28).
The results of a study by Rahmani et al. that evaluated the efficacy and immunity of INF-β-1b in the treatment of patients with severe COVID-19 indicated that the rate of ICU admission and the need for invasive mechanical ventilation using IFN-β-1b were significantly reduced in comparison to the control group. However, it did not reduce the length of hospital stay, length of stay in the ICU, intubation rate, and 28-day mortality, and there was no statistical difference between the two groups (14). The aforementioned studies are in accordance with the present study. However, the results of other studies that investigated the effect of INF compounds on COVID-19 have been contradictory (29-31). These discrepancies are probably due to the absence of a control group, the effects of drugs concomitant with INF, small sample sizes, or the lack of a standard treatment similar to the one used in the present study.
5.1. Study Limitations
Because this study was conducted in a referral center for COVID-19, based on the available information, about 60 - 70% of the patients referred to the emergency department of this hospital were in the severe phase of the disease upon admission to the hospital. Therefore, there were limitations in designing patient groups for mild, moderate, and severe cases, and it was impossible to compare the effectiveness of the drug in different groups. On the other hand, at the time of conducting this study, there was insufficient evidence about the effectiveness of INF compounds in mild or moderate cases of the disease. Therefore, it is necessary to conduct further studies with different groups and larger sample size.
Due to false negative PCR results in 20 - 40% of cases from different reports and given the high sensitivity of computed tomography to diagnose COVID-19, the patients who had epidemiological and radiological evidence compatible with COVID-19 with the approval of an infectious specialist were considered a case of illness.
Due to the limited number of ICU beds and a large number of patients at the time of this study, the researchers had to consider intubation rather than an ICU admission as one of the outcomes.
5.2. Conclusions
The use of INF-β-1b-containing treatment regimens in advanced and severe stages of COVID-19 does not reduce mortality and intubation rates. On the contrary, it might even increase the severity of the disease and the length of hospital stay for some patients. Therefore, it is not recommended to administer the INF drug in severe cases of COVID-19.