1. Introduction
Mucormycosis is a rare serious fungal infection associated with increased mortality. The incidence of mucormycosis has increased, especially in immunosuppressed cases, including patients with diabetes mellitus, hematological and solid organ malignancies receiving chemotherapy drugs, and patients receiving corticosteroids. The clinical manifestation depends on the site of infection, and the most commonly infected organs are the sinus, brain, lung, and skin (1). A primary gastrointestinal (GI) mucormycosis is a rare form of this fungal infection reported only in seven percent of patients with mucormycosis (2). Here, we present an ileocecal perforation due to mucormycosis in a patient with poorly controlled diabetes mellitus and acute myeloid leukemia (AML).
2. Case Presentation
2.1. Before Surgery
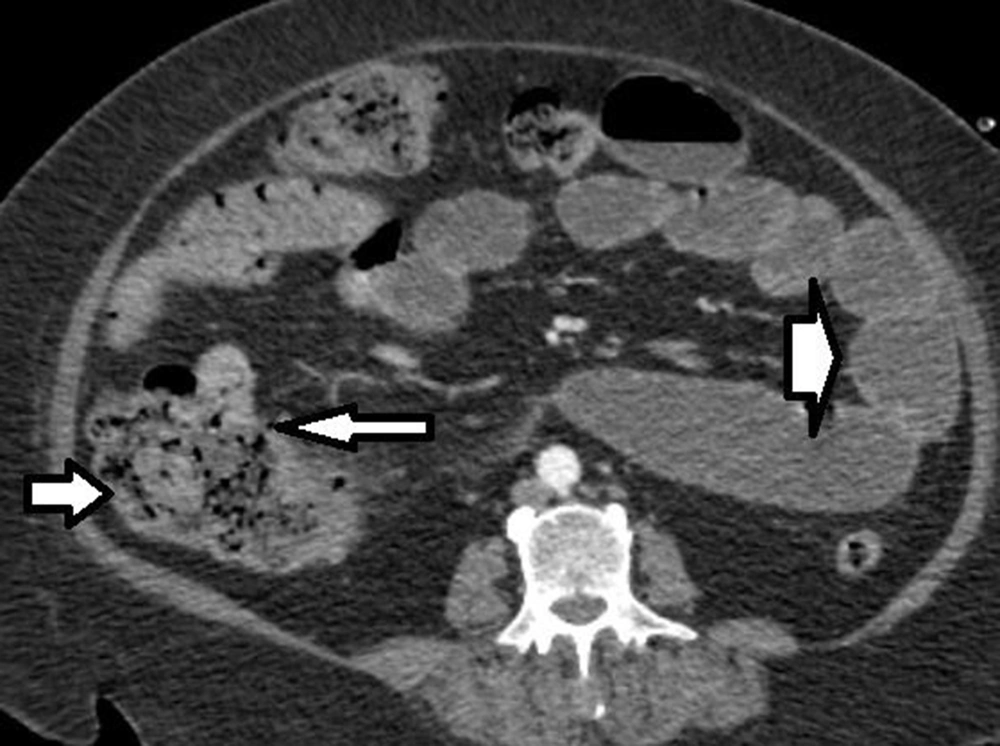
The patient was a 56-year-old woman diagnosed with AML and admitted to the hospital for chemotherapy. Initial examination revealed blood pressure of 135/90 mmHg, pulse rate of 80 beats/minute, O2 saturation of 92%, and body mass index (BMI) of 40 kg/m2. Past medical histories were hypertension and diabetes mellitus. She received insulin, dexamethasone 8 mg, and chemotherapeutic drugs, including Cytosar and Idarubicin. The laboratory findings on admission are shown in Table 1. She presented diarrhea without abdominal pain during chemotherapy, which was treated with diphenoxylate and loperamide. Thirteen days after admission, she complained of no defecation lasting eight days, and a week later, the patient presented sudden, severe, and generalized abdominal pain without any other symptoms. After 38 hours, she developed a poor general condition, abdominal guarding, and electrolyte imbalance. An abdominal computed tomography (CT) scan with IV contrast showed ileocecal necrosis with small bowel loop distention (Figure 1), so she was transferred to the operating room. The last laboratory data before surgery are mentioned in Table 2.
| Measure | Result |
|---|---|
| WBC (/μL) | 6300 |
| Neut (%) | 65 |
| RBC (× 106/μL) | 2.69 |
| Hb (g/dL) | 7.5 |
| PLT (/μL) | 70000 |
| Blood Sugar (mg/dL) | 349 |
| ESR (mm/h) | 89 |
| RDW (%) | 19.5 |
Laboratory Findings on Admission
| Measure | Result |
|---|---|
| AST (U/L) | 22 |
| ALT (U/L) | 25 |
| Bilirubin (mg/dL) | 0.7 |
| BUN (mg/dL) | 10 |
| Cr (mg/dl) | 1.4 |
| LDH (U/L) | 747 |
| Ca (mg/dL) | 6.8 |
| Mg (mEq/L) | 1.7 |
| Na (mEq/L) | 146 |
| K(mEq/L) | 4.1 |
| Alb (g/dL) | 2 |
| INR | 1.49 |
| WBC (/μL) | 16500 |
| Neut (%) | 88 |
Laboratory Data Before Surgery
2.2. Intraoperative Findings
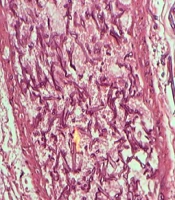
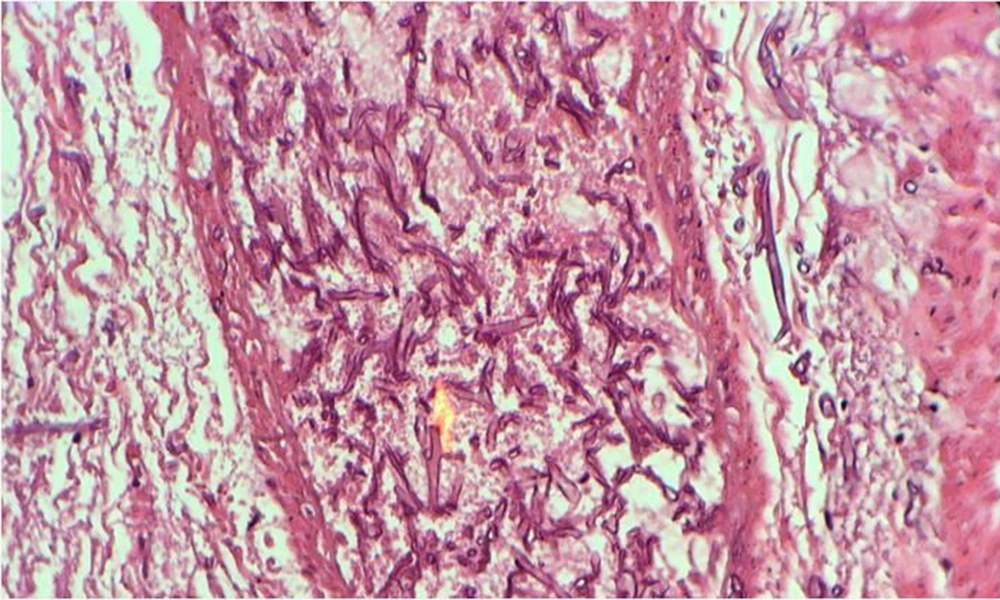
During surgery, fecaloid peritonitis with purulent secretion was seen in the abdominal cavity. Two separate perforations in the anti-mesenteric site of the terminal ileum (2 cm and 10 cm from the ileocecal junction) and cecum necrosis were seen. According to cecal necrosis and terminal ileum perforation, a right hemicolectomy was performed, and an end ileostomy was created because of the patient's hemodynamic instability. Immediately after the operation, the patient underwent broad-spectrum empirical antibiotic treatment with imipenem, vancomycin, and caspofungin. Unfortunately, the patient died five days after surgery due to septicemia and multi-organ failure. The histopathologic examination of the resected bowel confirmed necrotic tissue and revealed mucormycosis hyphae (Figure 2).
3. Discussion
Mucormycosis (caused by organisms of the class Zygomycetes) is the third most important invasive fungal infection after candidiasis and aspergillosis in patients with hematological cancers and has emerged as a fatal infection among these patients (1). Several organs are targeted by Mucormycetes, including the brain, lung, skin, kidney, and GI tract, resulting in devastating clinical long-term sequelae (2). The fungus spreads mainly by inhaling airborne spores, eating contaminated food, and using open wounds or skin cuts (3). The spores damage the endothelial cells and invade blood vessels leading to vascular thrombosis, perforation, bleeding, and tissue necrosis (1).
Gastrointestinal mucormycosis is an uncommon disease, even in immunosuppressed patients (2). The stomach is the most frequent GI organ affected by mucormycosis, followed by the colon and small intestine (4). The presenting symptoms of GI mucormycosis vary, and the most common one is abdominal pain. Nausea, vomiting, hematemesis, hematochezia, and melena are the other clinical manifestations of the disease (5). The mortality after GI involvement is often due to perforation, peritonitis, and GI bleeding (1).
Early diagnosis and treatment of mucormycosis are associated with better outcomes and reduced mortality. Moreover, it may decrease the need for surgery and other complications (2). Stool antigen tests, tissue sampling during colonoscopy, and new molecular methods are the recommended diagnostic tools for mucormycosis (1). However, according to the low specificity of these methods and non-specific symptoms of mucormycosis, reducing modifiable risk factors such as controlling blood sugar and decreasing corticosteroid use are the most beneficial management options for mucormycosis.
In summary, we reported a 56-year-old woman with a past medical history of diabetes mellitus presenting with abdominal pain and constipation. She was diagnosed with AML a month before surgery and received Cytosar, idarubicin, and dexamethasone 8 mg, which cause immunosuppression. The histopathological examination of the colon and ileocolic vessels showed mucormycosis infection. Since the patient had undergone chemotherapy, the immune system was suppressed, and the cecum region was the site of injury and necrosis, typhlitis with mold superinfection was the patient's first diagnosis. Unfortunately, the patient passed away five days after surgery because of septicemia due to bowel perforation. The coexistence of immunosuppressive diseases like diabetes mellitus and receiving corticosteroids in combination with chemotherapy drugs may increase the risk of mucormycosis. Therefore, more caution should be taken in administering these drugs to patients with underlying diseases.