1. Background
Hydatid cyst (HC) or Cystic Echinococcosis (CE) is a chronic disease, caused by the larval stages of tapeworms of the genus Echinococcus (1). The life cycle of Echinococcus parasites is completed in two hosts. The final hosts of adult worms are commonly the small intestines of canines. Some animals and humans play the role of intermediate hosts in the life cycle of these parasites and larval stages (or CE) formed in different parts of the body (1). The World Health Organization (WHO) described this disease as a “neglected tropical disease.” Also, evidence shows that CE can be life-threatening in humans, imposing high medical costs and economic burdens (2, 3).
The standard treatment for CE is surgery, which is both aggressive and high-risk; besides, this approach is expensive and has several side effects. Some negative consequences of this approach include ruptured cysts, the release of protoscolices, and the formation of secondary cysts, leading to the relapse of CE (4). On the other hand, cysts that do not require surgical treatment can be treated with medicines. The most important medicines against CE include albendazole and mebendazole. These medicines are sometimes used as the main treatment, although they are commonly used as a complementary approach to control and prevent the recurrence of HC after surgery (5).
To treat and prevent the spread of hydatid cysts in the body, researchers have focused on finding new medicines with antiparasitic effects and new immunomodulatory agents. Besides, the development of new drugs to prevent the formation of new cysts or the recurrence of disease has been always highlighted in research activities. So far, the effects of various medicines and plant-derived substances on protoscolices have been evaluated. In this regard, a study examined the impact of albendazole and mebendazole on the survival rate of protoscolices in vitro and showed that albendazole led to the death of all protoscolices in a shorter period (30 days) than mebendazole (6). Moreover, a comparison of the anti-protoscolices effects of Artemisia sieberi and albendazole indicated the faster protoscolicidal effect of Artemisia sieberi essential oil than albendazole (7).
As an antiepileptic drug, Valproic acid or valproate (VPA) is extensively prescribed to treat generalized and partial seizures worldwide. This drug is reported to have numerous effects on nerve tissues. It has also shown significant activity against epilepsy and other brain diseases, for which no specific mechanism has been established. As cellular and molecular factors underlie different types of seizures, the antiepileptic effect of VPA involves a combination of chemical and physiological mechanisms in nerve tissues (8).
In an in vitro study, a researcher’s mistake resulted in the identification of the antimicrobial and antifungal characteristics of VPA (9). Additionally, the inhibitory effects of VPA on Toxoplasma proliferation and relapse of seizure due to cerebral hydatid cysts have been confirmed (10, 11). Because hydatidosis is a complex and chronic disease, there is still no strong evidence to approve the available treatment options (4). Therefore, research investigating the effects of various drugs and compounds on these parasites is ongoing to introduce alternative drugs with minimal side effects and maximal therapeutic efficacy.
2. Objectives
For many years, VPA has been used as a safe drug in humans. Although its antiparasitic effects have been investigated in some studies, no research has yet examined its effects on hydatid cysts. Therefore, the current study aimed to examine the protoscolicidal and tissue destruction properties of VPA in vitro.
3. Methods
3.1. Parasite Preparation
Liver hydatid cysts were obtained from a slaughterhouse and transferred to the parasitology laboratory. A syringe was used to drain HC content under sterile conditions. The supernatant was discarded after protoscolices precipitation in a sterile glass container for half an hour. Then, protoscolices were washed in triplet using phosphate-buffered saline (pH = 7.2).
Eventually, a suspension of 9,000 to 10,000 protoscolices/mL was prepared in RPMI. Afterward, suspensions with 90% alive protoscolices were transferred to dark containers and kept at 4°C (12, 13). Following the removal of hydatid cysts, cystic layers, which contained a laminated and a germinal layer, were isolated from the host tissue (sheep). Finally, the layers were washed with phosphate buffer for histological investigations.
3.2. Experimental Design
Valproate was prepared at concentrations of 25, 50, and 75 mg/mL in an RPMI culture medium. The experiments were performed on seven groups (based on exposure to different substances), each including five subgroups (based on exposure time). Next, 500 μL of VPA (25, 50, and 75 mg/mL), RPMI (VPA solvent), mebendazole (5 and 100 µg/mL), or 0.001% DMSO (mebendazole solvent) was added to separate Eppendorf tubes; parasite sediments containing 500 protoscolices were then added to each tube. The protoscolices were exposed to the mentioned substances for 30, 60, 120, 180, and 240 minutes. Following each interval, dead protoscolices were counted in 100 protoscolices. The mortality rate (MR) of protoscolices was evaluated by 0.1% eosin staining, and the activity of flame cells was examined under a light microscope. Live protoscolices were colorless, while dead protoscolices were red. The tests were repeated in triplicate. Also, the MR of protoscolices, exposed to RPMI and DMSO, was considered as the control.
3.3. Histological Study
A similar design was used to determine the effects of mebendazole and VPA on the HC wall. Then, 10% formalin was used to fix the layers of HC for 48 hours, followed by washing the tissue using saline. Preparation of tissue comprised of fixation, preparation of paraffin blocks, tissue cutting, and hematoxylin-eosin (H&E) staining of each tissue. Finally, the sections were investigated and visualized under a light microscope (Nikon, Japan). The chemicals and compounds were purchased from Merck Co. (Germany) (14).
3.4. Immunohistochemistry for Caspase-3 Detection
A rabbit polyclonal antibody (AB4751, Abcam Co., UK) was used to perform immunohistochemistry, according to the kit manufacturer’s instructions (15).
3.5. Statistical Analysis
Data analysis was done using Excel 2019 and SPSS 23. The Kolmogorov-Smirnov test and Shapiro-Wilk test were applied to test for a normal distribution. Moreover, differences between the subgroups and control groups (RPMI and DMSO) were investigated using the repeated-measures test and Tukey’s Honestly Significant Difference (HSD) test. Statistical significance was considered when P < 0.05.
4. Results
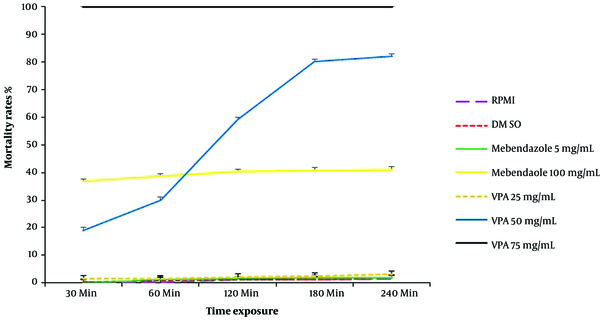
The mortality rates of protoscolices, exposed to RPMI, DMSO, mebendazole (two concentrations), and VPA (three concentrations), are provided in Figure 1. The Mortality Rate (MR) of protoscolices was directly related to the concentrations of mebendazole and VPA and exposure time. Also, high concentrations of mebendazole (100 µg/mL) and VPA (75 mg/mL) led to 36% and 100% mortality of protoscolices within 30 minutes, respectively.
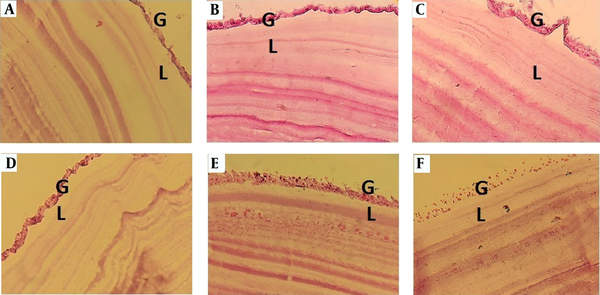
Investigation of protoscolices mortality revealed that the MR of protoscolices exposed to 50 and 75 mg/mL of VPA was significantly different from that of parasites exposed to mebendazole (5 and 100 µg/mL) and the control groups (RPMI and DMSO) (P < 0.001). The assessment of histological changes in the HC wall showed that in hydatid layers, exposed to RPMI and DMSO (control groups) and also 100 µg/mL of mebendazole, the laminated layer was clear and uniform. In addition, the structure of the germinal layer was clear, and the nuclei were visible. Conversely, in tissues exposed to VPA, the germinal and laminated layers were irregular or disappeared (Figure 2).
Histological changes in the HC wall following 120 minutes of exposure to A, RPMI; B, 0.001% DMSO; C, mebendazole (100 µg/mL); and D, E and F, 25, 50, and 75 mg/mL of valproate (VPA), respectively. A and B, The germinal layer is clear with visible nuclei, and the laminated layer is uniform and clear; C, The germinal and laminated layers are clear, but the germinal layer is irregular; D, The germinal layer is clear with visible nuclei, and the laminated layer is irregular; E, The nuclei of the germinal layer disappeared; the laminated layer is rough and irregular; F, The germinal layer is unclear, while the nuclei disappeared (40× magnification).
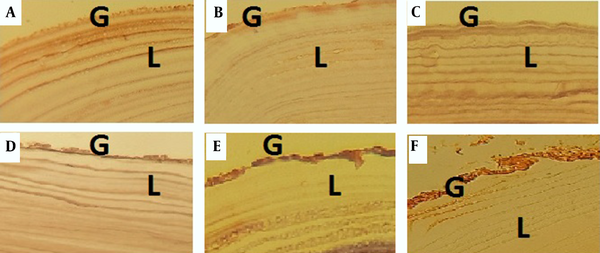
Investigating the activity of caspase-3 in HC walls via immunohistochemistry indicated its activity following exposure to mebendazole and VPA (Figure 3).
5. Discussion
This research showed the promising effects of VPA against protoscolices of E. granulosus. In this study, 75 mg/mL of VPA led to 100% mortality of protoscolices within one minute, and a VPA concentration of 50 mg/mL led to 82% mortality within 240 minutes. Some studies have investigated the effectiveness of VPA in the treatment of neurological symptoms caused by CE (11, 16, 17). However, the effect of VPA on protoscolices and hydatid cysts has not been examined so far. The current study is the first report on the impact of VPA on protoscolices of E. granulosus.
The effects of some medical treatments, plant extracts (aqueous or alcoholic), and essential plant oils on protoscolices and HCs have been studied in vitro and in vivo (4, 18-20). Some studies confirmed the effects of these drugs and plant derivatives on protoscolices in vitro, while others reported the therapeutic effects of some of these compounds in vivo (4, 18-20). Besides, the effects of some natural compounds, such as honey and vinegar, on these parasites have been evaluated and approved in vitro (21, 22). The WHO only recommends albendazole and mebendazole for CE treatment (23). These benzimidazole drugs are used to treat many parasitic worm diseases. A systematic review of the pharmacological treatment of echinococcosis showed that both drugs were effective for HC treatment; nevertheless, the treatment period was long (4). In this research, a comparison of the scolicidal effects of the highest concentrations of mebendazole and VPA in the longest exposure time (240 minutes) showed a mortality rate of 41% for protoscolices following exposure to 100 μg/mL of mebendazole. In contrast, the MR of protoscolices was 100% in exposure to 75 mg/mL of VPA; even a VPA concentration of 50 mg/mL led to the death of 82% of protoscolices.
Liu et al. (2014, 2015) found that the MR of protoscolices, exposed to 10 μg/mL of mebendazole during 24 hours, was 86%. These studies also showed that the incubation of protoscolices with higher concentrations of mebendazole in a longer exposure time increased the mortality of protoscolices, which is consistent with the present results (23, 24).
The lack of available drug options to treat CE has led researchers to propose and evaluate different and new drug classes to find alternative options (19). Valproate has been extensively administered as an antiepileptic drug for more than four decades (8). Nevertheless, in the last two decades, researchers have paid particular attention to its antimicrobial and anticancer properties (9, 25, 26). The findings of the current study demonstrated that mebendazole and VPA affected the protoscolices and HC walls; however, the effect of VPA was more rapid.
One of the causes of unsuccessful treatment of HC disease is the low drug concentration in the plasma and hydatid cysts (27, 28). Since VPA shows more solubility than mebendazole, its level in the plasma and HC may be adequate for treatment (8). Therefore, it is necessary to design and conduct further in vivo studies to obtain more accurate results.
5.1. Conclusion
Based on the present results, VPA, as a new protoscolicidal and anti-hydatid agent, can be a promising therapeutic option. However, further studies are needed to evaluate various characteristics of this medicine and confirm its effects in vivo.