1. Background
COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has resulted in significant mortality and morbidity all around the world. To date, there is no proven treatment for this disease. The recent development of vaccines to prevent SARS-CoV-2 infection gave us a glimmer of hope for overcoming this global issue (1). Nevertheless, there are ongoing discussions about the possible adverse effects of these vaccines and their efficacy, particularly about their administration for people with an underlying chronic disease. Persons with hemophilia are among those patients whose vaccination may be debatable.
Cases with hemophilia have no priority compared to the general population for SARS-CoV-2 vaccination, and they will be vaccinated based on the risks related to their general state of health, age group, and occupation (2). However, the impact of the COVID-19 pandemic on the availability and accessibility of laboratory and treatment facilities for patients with hemophilia makes their vaccination a necessity (3).
Due to the potential risk of bleeding following intramuscular injection in cases with hemophilia, the subcutaneous route is the principal recommended method of vaccination in this group of patients (4). However, currently, intramuscular administration is the only approved route of injection for available anti-SARS-CoV-2 vaccines (4). On the other hand, few reports of acquired hemophilia following SARS-CoV-2 vaccination are available, which caused concerns about the aggravation of bleeding complications in hemophilic people (5).
2. Objectives
To the best of our knowledge, there is no documented data on the safety of the Sinopharm COVID-19 vaccine for cases with hemophilia. Therefore, in this work, we evaluated the possible short-term vaccine-related adverse events in this group of patients.
3. Methods
We performed a cross-sectional study on all adult patients with hemophilia (types A and B) who were vaccinated with the Sinopharm COVID-19 vaccine in Afzalipour Hospital (a referral center located in Kerman city, Iran) from May to June 2021.
Study participants received two doses of the Chinese Sinopharm COVID-19 vaccine intramuscularly (0.5 mL in each dose) with an interval of three weeks. Vaccination was done using an auto-disable needle. After intramuscular administration of the vaccine, a local compression for about 10 minutes was applied at the site of injection. None of the patients received a prophylactic dose of coagulation factors before vaccination. Patients were educated for self‐examination (inspection and palpation) of the injection site for any evidence of bleeding or hematoma formation.
Included cases were followed for two weeks after receiving the 1st and 2nd doses of the vaccine for some possible vaccine-related adverse reactions. All included cases participated in the study with informed consent.
4. Results
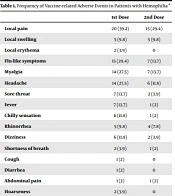
Overall, 51 persons with hemophilia were included (33 men (64.7%) and 18 women (35.3%)). Participants’ mean age was 37.07 ± 11.45 (19 to 67) years old. 27 (61.4 %) patients experienced at least one adverse reaction following vaccination. Local pain at the injection site was the most frequently reported adverse event, and 20 (39.2%) and 15 (29.4%) patients reported this complaint after the 1st and 2nd doses, respectively. Flu-like symptoms, headache, and myalgia were the most frequent systemic adverse events following both doses of the vaccine. Data are summarized in Table 1.
| 1st Dose | 2nd Dose | |
|---|---|---|
| Local pain | 20 (39.2) | 15 (29.4) |
| Local swelling | 5 (9.8) | 5 (9.8) |
| Local erythema | 2 (3.9) | 0 |
| Flu-like symptoms | 15 (29.4) | 7 (13.7) |
| Myalgia | 14 (27.5) | 7 (13.7) |
| Headache | 14 (27.5) | 6 (11.8) |
| Sore throat | 7 (13.7) | 2 (3.9) |
| Fever | 7 (13.7) | 1 (2) |
| Chilly sensation | 6 (11.8) | 1 (2) |
| Rhinorrhea | 5 (9.8) | 4 (7.8) |
| Dizziness | 6 (11.8) | 2 (3.9) |
| Shortness of breath | 2 (3.9) | 1 (2) |
| Cough | 1 (2) | 0 |
| Diarrhea | 1 (2) | 0 |
| Abdominal pain | 1 (2) | 1 (2) |
| Hoarseness | 2 (3.9) | 0 |
| Epistaxis | 1 (2) | 1 (2) |
| Urticaria | 0 | 1 (2) |
| Muscle spasm (neck) | 1 (2) | 1 (2) |
| Muscle spasm (upper extremity) | 0 | 1 (2) |
| Arthritis | 1 (2) | 0 |
| Menometrorrhagia | 2 (3.9) | 0 |
a Values are expressed as No. (%).
Two participants (3.9%) experienced menometrorrhagia following vaccine injection. One of them was admitted to the hospital and received one unit of packed red blood cells. One case (2%) had complaint of epistaxis. One patient (2%), who suffered from systemic lupus erythematous in addition to hemophilia, experienced arthritis of the knees following receipt of the 1st dose of vaccine.
During the follow-up period, no major cardiac event (including heart failure, cardiac arrhythmias, or myocarditis), acute liver or renal failure, any thromboembolic event, loss of smell, loss of taste, or mortality was reported. None of the study participants had a new documented infection with the SARS-CoV-2 virus within the follow-up period.
Data on vaccine-related adverse events, separated by gender, are provided in Table 2. As seen, complaints, including local pain (P-value: 0.003), headache (P-value: 0.045), and fever (P-value: 0.031), were significantly more frequent among female participants than males after injection of the 1st dose (Table 2).
| 1st Dose | 2nd Dose | |||||
|---|---|---|---|---|---|---|
| P-Value | Female | Male | P-Value | Female | Male | |
| Local pain | 0.003 a | 12 (60) | 8 (40) | 0.051 | 9 (60) | 6 (40) |
| Local swelling | 0.451 | 1 (20) | 4 (80) | 0.224 | 3 (60) | 2 (40) |
| Local erythema | 0.287 | 0 | 2 (100) | - | 0 | 0 |
| Flu-like symptoms | 0.346 | 6 (40) | 9 (60) | 0.689 | 2 (28.6) | 5 (71.4) |
| Myalgia | 0.176 | 7 (50) | 7 (50) | 0.193 | 4 (57.1) | 3 (42.9) |
| Headache | 0.045 a | 8 (57.1) | 6 (42.9) | 0.442 | 3 (50) | 3 (50) |
| Sore throat | 0.689 | 2 (18.6) | 5 (71.4) | 0.287 | 0 | 2 (100) |
| Fever | 0.031 a | 5 (71.4) | 2 (28.6) | 0.171 | 1 (100) | 0 |
| Chilly sensation | 0.087 | 4 (66.7) | 2 (33.3) | 0.171 | 1 (100) | 0 |
| Rhinorrhea | 0.224 | 3 (60) | 2 (40) | 0.521 | 2 (50) | 2 (50) |
| Dizziness | 0.915 | 2 (33.3) | 4 (66.7) | 0.657 | 1 (50) | 1 (50) |
| Shortness of breath | 0.657 | 1 (50) | 1 (50) | 0.456 | 0 | 1 (100) |
| Cough | 0.171 | 1 (100) | 0 | - | 0 | 0 |
| Diarrhea | 0.456 | 0 | 1 (100) | - | 0 | 0 |
| Abdominal pain | 0.456 | 0 | 1 (100) | 0.171 | 1 (100) | 0 |
| Hoarseness | 0.456 | 0 | 1 (100) | - | 0 | 0 |
| Epistaxis | 0.456 | 0 | 1 (100) | 0.456 | 0 | 1 (100) |
| Urticaria | - | 0 | 0 | 0.456 | 0 | 1 (100) |
| Muscle spasm (neck) | 0.171 | 1 (100) | 0 | 0.171 | 1 (100) | 0 |
| Muscle spasm (upper extremity) | - | 0 | 0 | 0.171 | 1 (100) | 0 |
| Arthritis | 0.171 | 1 (100) | 0 | - | 0 | 0 |
| Menometrorrhagia | 0.171 | 1 (100) | 0 | - | 0 | 0 |
a Data with significant difference (P-value less than 0.05).
To the best of our knowledge, this is the first report on the short-term safety of the Sinopharm COVID-19 vaccine and its related adverse reactions in patients with hemophilia. We found that local pain at the site of vaccine injection, either after the 1st dose or following the 2nd dose, was the most frequent adverse event. Overall, three patients experienced bleeding tendency in the forms of epistaxis and menometrorrhagia.
Previous phase one and two clinical trials, which intended to evaluate the safety of inactivated Sinopharm COVID-19 vaccine, reported an incidence rate of 15% for adverse events among all participants. According to these trials, mild and self-limiting injection site pain was the most common adverse reaction (6). Similarly, another cross-sectional study from UAE reported pain at the site of vaccine injection (42.2% of participants) as the most common side effect (7). Our research showed a similar finding in hemophilic patients, as local pain was the most frequent side effect.
Hemorrhagic complications are the main concern regarding the use of COVID-19 vaccines in persons with hemophilia. These complications can be divided into local (ie, hematoma formation following intramuscular injection of vaccine) and systemic. These concerns are intensified when we see previous case reports of acquired hemophilia following the administration of anti-SARS-CoV-2 vaccines or SARS-CoV-2 infection (8). Farley et al. reported a 67-year-old African-American male with acquired hemophilia type A whose complications presented about 19 days after injection of 2nd dose of Pfizer-BioNTech anti-SARS-CoV-2 vaccine. This case was complicated with a large hematoma of his left lower extremity, and a small ecchymosis on his right upper extremity. His laboratory investigations showed an abnormal and elevated activated partial thromboplastin time (aPTT) (9). Another case was reported by Radwi and colleagues, whose bleeding tendency presented about nine days after injection of the 1st dose of Pfizer-BioNTech SARS CoV-2 mRNA vaccine (10). Development of acquired hemophilia A is related to the formation of autoantibodies against clotting factor VIII (10). Wang et al. reported another 65-year-old male patient whose presentation was compartment syndrome secondary to severe subcutaneous bleeding of the arm. Laboratory investigations of this patient revealed acquired hemophilia type A following an asymptomatic SARS-CoV-2 infection (11). In our study, none of the patients experienced local hematoma formation at the site of vaccine injection. However, two cases experienced menometrorrhagia, and one patient had complaint of epistaxis.
It is necessary to mention some limitations of our study, including not evaluating changes in serum levels of coagulation factors 8 and 9 following vaccination, particularly for participants who experienced hemorrhagic adverse events and a short follow-up period.
In summary, according to our results, use of the Sinopharm COVID-19 vaccine in patients with hemophilia seems to be safe regarding bleeding adverse events. Further studies with larger sample size and longer follow-up period are needed to get a closer look at the possible side effects of this vaccine in hemophilic patients.