1. Background
Staphylococcus aureus is a Gram-positive pathogenic bacterium and an important nosocomial pathogen (1). Infections caused by S. aureus range from skin infections to systemic infections (2), such as purulent infections, staphylococcal pneumonia, scaly skin syndrome, and toxic shock syndrome (3). The success of S. aureus as a pathogen can partially be attributed to its antibiotic resistance and ability to form biofilms (4). Biofilms reduce the penetration of antimicrobial agents due to their special structure and the presence of extracellular polymeric materials. It is difficult to treat infections caused by biofilm-producing bacteria, and physicians are facing numerous problems in this case (5).
The first step in S. aureus infection is access to a variety of materials, including medical devices and host tissue. These reactions have been attributed to a combination of extracellular factors, such as adhesion and biofilm formation (6). Poly-N-acetylglucosamine (PNAG) is regarded as the primary exopolysaccharide of the S. aureus biofilm matrix, also recognized as polysaccharide intercellular adhesin (PIA). The PIA/PNAG is synthesized and accumulated on the cell surface through the products of intercellular adhesin (ica) genes. Operon ica consists of icaADBC, with four genes (7). The first two genes, icaA, and icaD, have the main role in synthesizing exopolysaccharides (8). A transmembrane protein with N-acetyl-glucosaminyl transferases enzymatic activity is the product of the icaA gene resulting in the synthesis of the poly-N acetyl glucosamine polymer (9). Based on the evidence, the icaD gene product is necessary for the most beneficial enzymatic activity of the product of the icaA gene (9, 10).
The first example of a surface protein that is able to induce biofilm development is biofilm-associated protein (bap). It has two areas that are able to bind to calcium and control the function of bap in response to calcium in the growth medium. In bacteria, calcium is involved in a variety of important processes, such as cell cycle, cell division, pathogenesis, and motility. It also plays an important structural role in the extracellular space and maintains the integrity of the outer lipid layer and the cell wall (11, 12).
One way to form biofilm is by using herbal essential oils instead of antibiotics. Herbal essential oils are complex compounds of different amounts of various chemical components. Medicinal plants are also mostly available and affordable (13). Thymus vulgaris is a plant from the mint family. Thyme essential oil has antibacterial and antifungal properties and is an antioxidant and a natural food preservative (14). Thyme essential oil can prevent the growth of Escherichia coli enterohemorrhagic, S. aureus, Enterococcus, Pseudomonas aeruginosa, Acinetobacter baumannii, and Alacaligenes (6, 15). The antimicrobial properties of whole essential oils are greater than any of their compounds, indicating the synergistic composition of essential oils (16).
Cinnamomum verum is an aromatic tree species belonging to the Lauraceae family. Cinnamon is obtained from the bark of young branches of the cinnamon tree, which is used worldwide due to its aroma, flavor, and taste (17). The different parts of the plant are used in Indian traditional medicine for antibacterial, antifungal, and anti-inflammatory purposes (18-20). Cinnamon has important antibacterial activity against E. coli, Salmonella Typhimurium, and S. aureus (21).
2. Objectives
This study aimed to evaluate the effect of Thymus vulgaris and Cinnamomum verum essential oils on biofilm formation and expression of icaA, icaD, and bap genes in S. aureus strains.
3. Methods
3.1. Bacteria Isolates
In this study, 84 clinical urine specimens were collected from hospitalized patients in the intensive care unit of Taleghani hospital in Tehran, Iran. The specimens were cultured on Blood agar and incubated at 37°C for 24 hours. Fresh cultures were used for staining and differential tests, such as catalase, coagulase, gelatinase, and culture on mannitol salt agar. Accordingly, 20 S. aureus strains were isolated. In these experiments, a standard biofilm producer strain S. aureus ATCC25923 was used as a control.
3.2. Ingredients of Thyme and Cinnamon Essential Oils
Thyme and cinnamon essential oils were purchased from Golha Teb Kashan Company (Golha Teb, Kashan, Isfahan, Iran). The analysis of the compounds of thyme and cinnamon essential oils was performed by gas chromatography-mass spectrometry. Thymol with 33.33% and carvacrol with 28.69% constitute the most components of thyme essential oils. Cinnamaldehyde, with 56.3%, was the most important ingredient in cinnamon essential oil.
3.3. Minimum Inhibitory Concentration Test
The broth microdilution method, as described in the Clinical and Laboratory Standards Institute, was used for the antibacterial susceptibility test. A suspension of thyme essential oil with a concentration of 128 µg/mL was prepared, and serial dilutions were made into microdilution plates in 48 U-shaped wells (Nunc A/S Co., Roskilde, Sjaelland, Denmark). The bacterial suspensions of S. aureus strains in the Mueller-Hinton broth medium were adjusted to 106 CFU/mL (1/100 McFarland standards turbidity), and 100 μL aliquots of this were added to all wells containing diluted thyme essential oil. A positive control well (culture medium and bacterial suspension) and negative control well (culture medium and essential oil) were used for each plate. The plates were incubated at 37°C for 24 hours. All the aforementioned steps were performed for cinnamon essential oil. Finally, the growth rate of bacteria was assessed by the turbidity of wells by an ELISA reader (BioTek Co., Winooski, Vermont, US) at the wavelength of 620 nm.
3.4. Effect of Minimum Inhibitory Concentration of Thyme and Cinnamon Essential Oils on Biofilm Formation
The biofilm formation of S. aureus strains was evaluated by the microtiter plate method. In this regard, 100 µL of minimum inhibitory concentration (MIC) of thyme essential oil was added to microtiter plates. The suspension of all S. aureus strains was made in Tryptic soy broth medium supplemented with 2% glucose adjusted to 108 CFU/mL (McFarland standards turbidity) added to the wells. The plates were incubated at 37°C for 24 hours. All the aforementioned steps were performed for cinnamon essential oil. Finally, the plates were stained, and biofilm formation was evaluated by optical density cut-off (ODc). In order to check, the calculation was performed according to the following formula:
ODc: Average optical density of negative control wells + (standard deviation of negative control wells × 3)
After calculating the optical density (OD) or cut-off, the OD of the studied wells was classified according to Table 1.
| Observed OD | Power of Biofilm Formation |
|---|---|
| OD > 4 × ODc | Strong biofilm |
| 2 × ODc < OD ≤ 4 × ODc | Moderate biofilm |
| ODc < OD ≤ 2 × ODc | Weak biofilm |
| OD ≤ ODc | Negative biofilm |
Power of Biofilm Formation
3.5. Ribonucleic Acid Extraction
The ribonucleic acid (RNA) extraction of S. aureus bacteria was performed to evaluate the expression of icaA, icaD, and bap genes for three control and treatment groups. The RNA extraction was performed by Qiagen (Qiagen Co., Nordrhein-Westfalen, Germany). The cultivated bacteria in the presence (treatment groups) and the absence (control group) of essential oils were collected in the tube and centrifuged for 5 minutes at 8000 g, and the supernatant was discarded. A volume of 200 μL of trisol solution was added to each vial, and then vortex was applied for 15 minutes. It was then incubated at room temperature for 10 minutes until the bacterial lysis was complete. Then, 200 μL of chloroform was added and mixed gently for 15 seconds and incubated for 5 minutes in a 4°C refrigerator. Then, it was centrifuged for 15 minutes at 4°C at 12000 g. At this stage, two phases were formed. The aqueous phase was transferred to a new RNase-free 1.5 mL RNase-free microtube, and the same volume, cold isopropanol, was added and mixed slowly. It was then placed at 4°C for 20 minutes. Subsequently, it was centrifuged for 15 minutes at 12000 g at 4°C, and the supernatant was discarded. Moreover, 1 mL of 70% ethanol was added to it and slowly vortexed for 3 to 5 seconds. It was then centrifuged for 8 minutes at 7500 g at 4°C. The ethanol was then discarded and allowed to dry at room temperature for a few minutes. The RNA pellet was dissolved in 50 μL of DEPC-treated water by incubating for 10 minutes at a 55 - 60°C water bath. The OD of the RNA was measured by a nanodrop (Thermo Scientific, Waltham, Massachusetts, US) at the wavelength of 260/280 nm, which should be within 1.8 and 2.8. The product was stored at -70°C and used for complementary deoxyribonucleic acid (cDNA) synthesis.
3.6. Estimation of Gene Expression by Real-Time Polymerase Chain Reaction
The estimation of differences in icaA, icaD, and bap gene expression in S. aureus was performed by quantitative real-time reverse-transcription polymerase chain reaction (RT-PCR). The cDNA synthesis was used according to the instructions of the manufacturer kit (GreenALL, Seoul, South Korea), and equal concentrations of RNA (1 μg in 20 μL) were subjected to cDNA synthesis using random hexamer primers and reverse transcriptase enzyme. This product was stored at -70°C and used for real-time RT-PCR.
The sequence of primers used in this study for icaA, icaD, and bap genes were identified as genes involved in biofilm production and 16S rRNA gene as internal control gene. The primer sequences were obtained from a published paper, and their sequences were examined with BLAST software (version 2020) in the National Center for Biotechnology Information (Table 2).
Nucleotide Sequences of Primers Used in Reverse Transcription-Quantitative Polymerase Chain Reaction Assay
Real-time RT-PCR was carried out using the SYBR green master mix (Applied Biosystems, Foster City, California, US) and an ABI thermocycler (Applied Biosystems, Foster City, California, US). The PCR conditions included 94°C for 5 minutes, 45 cycles of 95°C for 50 seconds, 58°C for 20 seconds, and 72°C for 30 seconds. The final extension was carried out at 72°C for 1 minute.
3.7. Gene Expression Analysis
Gene expression levels were determined by the 2-∆∆Ct method using REST software (version 2018). For the normalization of the data, 16S rRNA was used as a housekeeping gene. Statistical analysis was performed using SPSS software (version 22).
4. Results
4.1. Biofilm Formation by Microtiter Plate Method
In the present study, all strains of S. aureus showed the ability of biofilm formation. In this study, 75% and 25% of the strains had strong and moderate biofilms, respectively.
4.2. Determination of MIC of Thyme and Cinnamon Essential Oils in Staphylococcus aureus
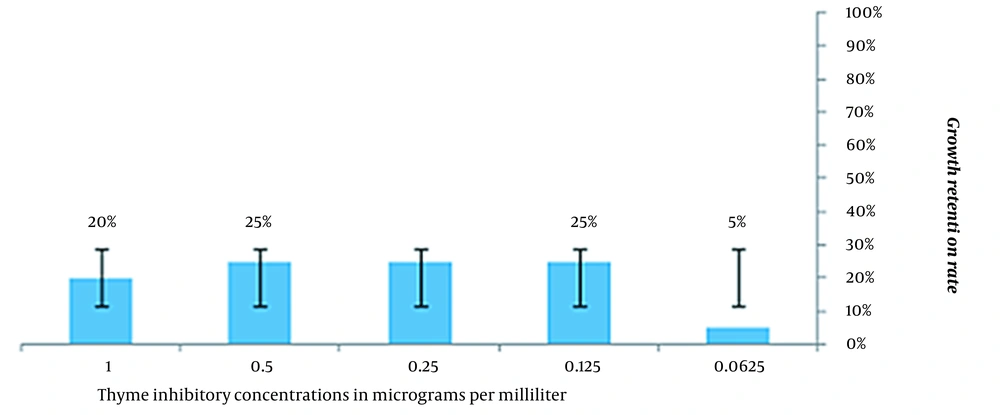
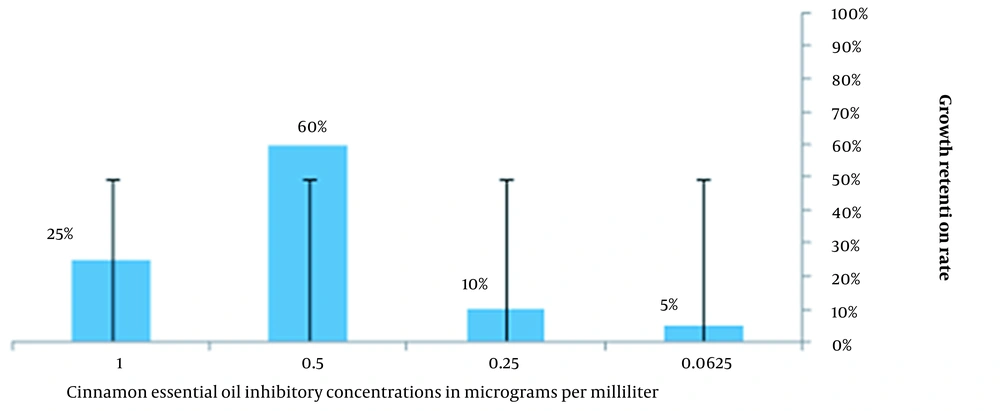
This study investigated the inhibition growth of thyme and cinnamon essential oils on all strains of S. aureus. The MIC is the lowest concentration of essential oils, which reduces the population of bacteria. Thyme essential oil concentrations of 0.5, 0.25, and 0.125 μg/mL were similar with a frequency of 25%, and a concentration of 0.5 μg/mL of cinnamon essential oil with a frequency of 60% maximum inhibition of growth was observed among the strains of S. aureus (Figures 1 and 2).
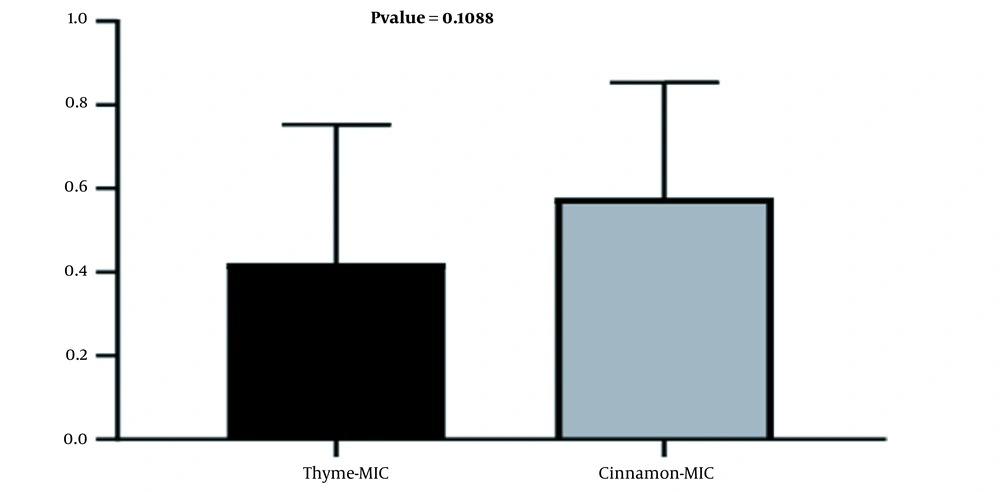
In the present study, there was no significant relationship between the growth inhibitory concentrations of thyme and cinnamon essential oils (Figure 3).
4.3. Effect of MIC of Essential Oils on Staphylococcus aureus Biofilm Formation
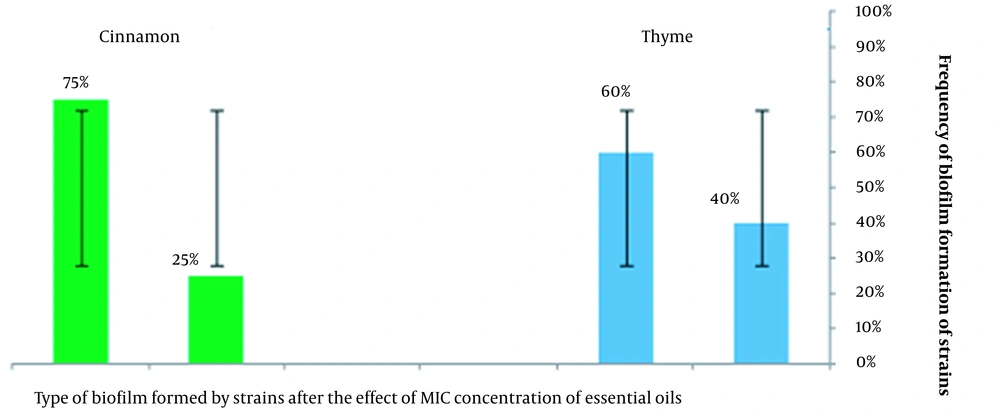
This study showed the biofilm formation of the S. aureus strains after treatment with the MIC of thyme essential oil (0.5 μg/mL) as a weak biofilm with 40% and negative biofilm with 60%, and after treatment with the MIC of cinnamon essential oil (0.5 μg/mL) as strong biofilm with 25% and moderate biofilm with 75% ( Figure 4).
4.4. Effect of Essential Oils on Gene Expression
For the evaluation of the expression of the above-mentioned genes, 20 S. aureus biofilm producer strains were selected, and the expression of icaA, icaD, and bap genes in the strains was evaluated by the effect of the MIC (0.5 μg/mL) of thyme and cinnamon essential oils. For the determination of the amplification efficiency of PCR in the real-time PCR technique, the melting curve and the logarithmic replication curve of the studied genes were examined using StepOne software (version 2018) of the ABI device.
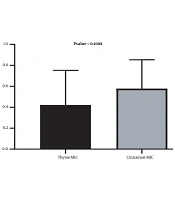
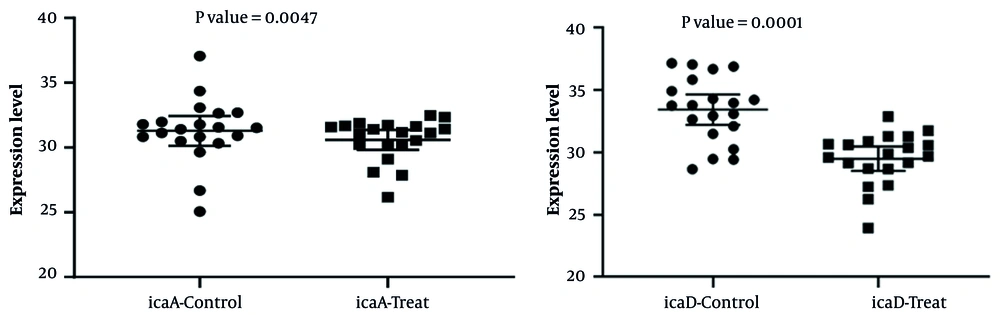
According to the calculation of gene expression by the fold change method, 60% and 55% of S. aureus strains treated with the MIC (0.5 μg/mL) of thyme essential oil, compared to their control group, showed a decrease in icaA and icaD gene expression, respectively. In the present study, a significant relationship was observed between decreased icaA and icaD gene expression in the control group and the group treated with thyme essential oil (Figure 5).
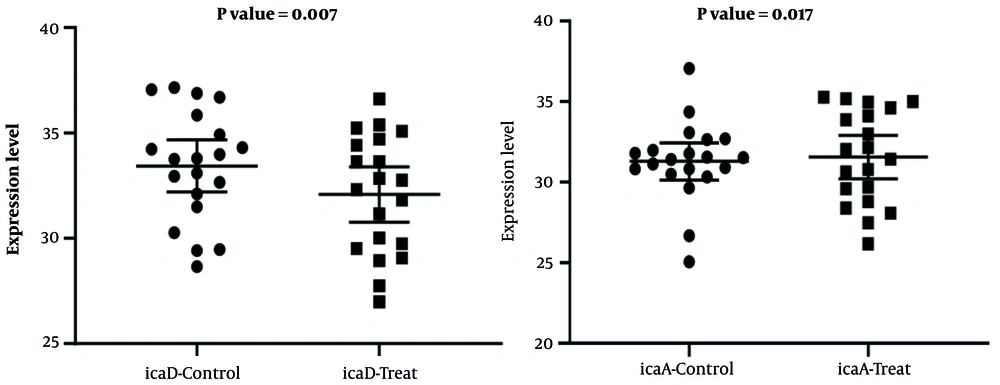
According to the calculation of gene expression by the fold change method, 60% and 70% of S. aureus strains treated with the MIC (0.5 μg/mL) of cinnamon essential oil, compared to their control group, showed a decrease in icaA and icaD gene expression, respectively. In the present study, a significant relationship was observed between decreased icaA and icaD gene expression in the control group and the group treated with cinnamon essential oil (Figure 6).
In this study, none of the studied strains of S. aureus had a bap gene.
5. Discussion
Staphylococcus aureus is able to cause disease according to its ability to produce a wide diversity of virulence factors contributing to bacterial invasion, including factors accountable for attachment, adherence, and biofilm formation (25). The PIA, synthesized from β-1, 6-linked PNAG, causes to initiate the biofilm matrix maturation into multi-layered patterns (26). The mediation of PIA synthesis is carried out by the ica locus that includes four core genes of icaA, icaD, icaB, and icaC and a regulatory gene (icaR) (27). The production of slime is facilitated by the expression of icaA and icaD genes (28). In addition, the bap is vital for the primary attachment of S. aureus and biofilm formation (29, 30). One way to combat biofilm is to use herbal remedies instead of antibiotics. The antimicrobial properties of plant essential oils are well-known. Due to the aforementioned biological effects, essential oils are considered suitable alternatives to antibiotics for therapeutic purposes (31).
The present study showed that 75% and 25% of the strains formed strong and moderate biofilms, respectively. The biofilm formation of the strains after treatment with the MIC of thyme essential oil was 40% weak and 60% negative and for cinnamon essential oil was 25% strong and 75% moderate. The effectiveness of various plants in preventing the formation of biofilms in various microorganisms, including S. aureus, has been proven (32, 33). The results of the present study showed that thyme essential oils (carvacrol and thymol) have a very good effect on preventing the formation of biofilms and changing the phenotype from strong and moderate biofilms to weak and negative biofilms.
In Kot et al.’s study, according to the results of the present study, it was stated that trans-cinnamaldehyde and thyme essential oils change the biofilm phenotype from strong to weak in the formation of methicillin-resistant Staphylococcus aureus (MRSA) biofilm (34). In another study by Ceylan and Ugur, the ability of biofilm formation in S. aureus, S. epidermidis, P. aeruginosa, and Pseudomonas fluorescens was evaluated by the microtiter plate method. It was also stated that thymol and carvacrol have antibiofilm properties on the aforementioned microorganisms (35).
Additionally, cinnamon essential oil (cinnamaldehyde) showed less effect on changing the phenotype of biofilm formation in MIC than thyme essential oil. Various studies showed that the high antibacterial activity observed in cinnamon essential oil might be due to the action of trans-cinnamaldehyde, considered its single major compound (36). Kot et al.’s study states that cinnamaldehyde has therapeutic potential by inhibiting infections related to biofilm production by S. aureus and P. aeruginosa (34). The results of Kerekes et al.’ study showed that trans-cinnamaldehyde significantly inhibited the biofilms of S. aureus, E. coli, and Listeria monocytogenes, and according to the results of the present study, cinnamon was less effective than thyme (37).
The results of the present study showed that thyme essential oil is 55 - 60% effective in reducing the expression of icaA and icaD genes (involved in the PIA pathway for biofilm formation). Considering that this essential oil changes the phenotype from strong and moderate biofilms to weak and negative biofilms, it can be concluded that in addition to the PIA-dependent pathway, it also plays a role in preventing biofilm formation in the pathway independent of the PIA. The cinnamon essential oil was also effective in reducing icaA and icaD gene expression by 60 - 70%. This essential oil showed less effect on changing the phenotype of biofilm formation in MIC than thyme essential oil but had a greater decrease in expression in icaA and icaD genes. Cinnamon essential oil further inhibits biofilm formation in the PIA-dependent pathway.
Thymol is one of the main components of thyme essential oils. Yuan et al. investigated the ability of thymol to inhibit biofilm formation and kill MRSA biofilm. Yuan et al. found that thymol inhibited and destroyed the MRSA biofilm by reducing PIA synthesis and reducing Extracellular DNA (eDNA) release (38). Since eDNA in the PIA-independent pathway causes the spread of S. aureus biofilm, it can be concluded that, according to the results of the present study, thyme inhibits biofilm formation by acting on the PIA-dependent and PIA-independent pathways.
Lui’s studies showed that Yunnan Baiyao (i.e., a Chinese drug derived from Panax ginseng) inhibits the lateral gene regulatory system (agr) and inhibits icaA, sarA, and messenger ribonucleic acid expression related cidA in the biofilm formation of S. aureus (39). Since agr, sarA, and cidA are involved in the formation of S. aureus biofilm in the PIA-independent pathway, it seems that Panax ginseng has a greater inhibitory effect on the PIA-independent pathway in the biofilm formation of S. aureus.
Shojaei Baghini et al.’s study examined the MRSA strain with icaD and icaA genes. The results showed that the combination of Artemisia aucheri and Artemisia oliveriana inhibits icaA and icaD genes and inhibits the formation of MRSA biofilm (40). According to Shojaei Baghini et al.’s study and the current study’s results, it can be concluded that Artemisia aucheri and Artemisia oliveriana act similarly to cinnamon essential oils and inhibit the biofilm of S. aureus by affecting the PIA-dependent pathway.
Topa stated in a study that cinnamaldehyde is able to inhibit quorum sensing. Therefore, it makes P. aeruginosa more susceptible to antibiotics and has synergistic effects in inhibiting the biofilm of this bacterium (41). Cinnamaldehyde is the main component of cinnamon essential oil which, according to the results of the present study, inhibits biofilm formation in S. aureus by acting on the PIA-dependent pathway. Contrary to the results of the present study, Topa concluded that cinnamaldehyde inhibits biofilm formation by affecting the pathway independent of the PIA.
Kot et al.’s studies showed that trans-cinnamaldehyde inhibited the formation of MRSA by reducing the expression of icaA and icaD genes (42). Since trans-cinnamaldehyde is an essential component of cinnamon essential oil, and icaA and icaD genes are PIA-dependent genes in the formation of S. aureus biofilm, according to the results of the present study, in the Kot et al.’s study, trans-cinnamaldehyde inhibits biofilm formation by affecting the PIA-dependent pathway.
5.1. Conclusions
The present study showed the effect of thyme and cinnamon essential oils on reducing the biofilm formation of S. aureus strains. Thymus vulgaris and Cinnamomum verum essential oils reduce the expression of icaA and icaD genes, and Cinnamomum verum essential oil is more effective than Thymus vulgaris essential oil. According to the results of this study, it was shown that Thymus vulgaris essential oil is effective in reducing the expression of icaA and icaD genes by 55 - 60%, which are the genes involved in the PIA pathway in the direction of biofilm formation. Considering that this essential oil showed a very good effect on preventing the formation of biofilm and changing the phenotype from a strong and moderate biofilm to a weak and negative biofilm, it can be concluded that this essential oil prevents the formation of biofilm In both PIA-dependent and PIA-independent pathway. Furthermore, the cinnamon essential oil was effective in reducing the expression of icaA and icaD genes by 60 - 70%, indicating that this essential oil showed less effect on changing the phenotype of biofilm formation in MIC than Thymus vulgaris essential oil, but a greater reduction in the expression of icaA and icaD genes. Therefore, it seems that cinnamon essential oil inhibits biofilm formation more in the PIA-dependent way, which again needs further investigation in this field.