1. Background
Magnesium (Mg), an essential element of life, is the fourth most abundant cation in the human body and the second most common cation in the intracellular compartment after potassium (1, 2). There is about 25 g or 1000 mmol Mg in healthy adult individuals, which includes nearly 60% stores in bones, 20% in soft tissues, 20% in muscles, 0.5% in red blood cells, and 0.3% in plasma (3). Mg is an essential mineral for the human body because it affects the activities of 300 enzyme systems, such as Na-K-ATPase-mediated transport. In addition, it is a crucial cation for calcium homeostasis, skeletal muscle function, conduction of nervous messages, and the regulation of potassium and calcium homeostasis (4, 5). Therefore, as an essential substance, Mg is needed in lower amounts for the proper function of critical metabolic and physiological processes (6).
Considering the vital role of Mg in maintaining homeostasis, its severe deficiencies are possibly life-threatening (6). Although hypomagnesemia is a common and important electrolyte abnormality in critically ill patients, it is frequently ignored. Critically ill patients admitted to the intensive care unit (ICU) due to various comorbidities, such as cancers, cardiovascular diseases, and renal failure, are potentially at an increased risk of Mg deficiency because of illness-induced reduced intake, increment losses, medicational interactions, and potentially increased needs (6, 7).
It has been reported that Mg deficiency in critically ill patients may lead to major clinical consequences, such as cardiac arrhythmias, hypocalcemia, and hypokalemia, as well as neurological and psychiatric complications, subsequently increasing morbidity and mortality (8, 9). Therefore, it is suggested that monitoring serum Mg in critically ill patients can have prognostic and perhaps therapeutic applications (8). The studies conducted to assess the Mg status and Mg therapy in critically ill patients were reviewed by Fairley et al. (7). According to this systematic review, some studies have reported a positive association between hypomagnesemia at ICU admission and higher mortality (10-13). Moreover, lower Mg level positively correlates with some comorbidities, such as abdominal aortic aneurysm surgery, high blood pressure, and sepsis (14, 15). Furthermore, it has been shown that hypomagnesemia is positively linked to the longer length of mechanical ventilation and ICU stay (11-13, 15, 16). However, there were heterogeneous and contradictory findings on the relationship between hypomagnesemia and hemodynamic, biochemical, and clinical outcomes (7).
2. Objectives
Monitoring and evaluating serum Mg may have prognostic and therapeutic implications in critically ill patients with various comorbidities. Therefore, in the current study, we aimed to investigate the possible difference in Mg levels among patients admitted to the ICU based on the mortality status (between subjects with and without mortality) and also various comorbidities status (including sepsis, kidney failure, and liver failure), as well as based on the need for mechanical ventilation and length of stay in the ICU. Using stratified analysis, we performed this investigation in patients based on COVID-19 infection status.
3. Methods
The current study was approved by the Research Committee of Hormozgan University of Medical Sciences. We conducted this research in the ICU of Shahid Mohammadi Hospital from June to December 2021. A total of 69 critically ill patients aged > 18 years admitted to the ICU were included in this study based on the inclusion and exclusion criteria. The serum Mg levels of patients included in the study were measured in the first 48 hours of hospitalization. Individuals were excluded from our analysis if they were in the pregnancy or lactation period. Patients with palliative care, intravenous feeding, or Mg supplements were excluded. Furthermore, the cases with acute renal failure or those on dialysis were excluded from this study.
In this study, 69 patients were followed up in the ICU for the occurrence of primary outcomes, namely death, sepsis, renal failure, liver dysfunction, or liver failure. Various secondary outcomes, including the duration of ICU hospitalization, length of mechanical ventilation, serum levels of albumin, calcium, and potassium, and scoring system of sequential organ failure assessment (SOFA) and acute physiology and chronic health evaluation II (APACHE II) were determined in all participants. We followed the participants until they were discharged from ICU.
A trained expert interviewer used a standard questionnaire to collect data on various variables, including age, gender, weight, height, medication use, and comorbidities history. The body mass index (BMI) was computed as weight (kg) divided by height (m). A 5 mL venous blood sample was taken from participants during the first 48 h of admission. The blood samples were transferred into vacutainer tubes and were centrifuged within 30 - 45 min of collection. All biochemical analyses were performed using a Selectra 2 auto-analyzer at the laboratory of Shahid Mohammadi Hospital on the day of blood collection. The serum level of Mg was measured using Xylidyl Blue photometric method (Pars Azmoon Inc., Tehran, Iran). The normal range for serum Mg is 1.5 - 2.5 mmol/L (4). The levels of albumin, calcium, potassium, aspartate aminotransferase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) were measured using the enzymatic colorimetric methods. Analyses were performed using commercial kits (Pars Azmoon Inc., Tehran, Iran). The scores of two scoring systems, namely SOFA (17) and APACHE II (18), were determined using a standard calculator previously explained in detail. Positive COVID-19 cases were individuals who had confirmed positive PCR tests for COVID-19 and had the symptoms of COVID-19, such as dry cough, gastrointestinal symptoms, pulmonary symptoms, fever, and a lack of sense of smell and taste.
3.1. Statistical Analysis
The statistical package for social sciences version 21.0 (SPSS Inc, Chicago, IL, USA) was used to analyze the data. We checked the normality of variables using histogram charts and the Kolmogorov-Smirnov test. The chi-square test and independent two-sample t-test were used to compare the qualitative and quantitative variables in patients based on mortality or comorbidities status classification, respectively. The characteristics of patients were expressed as mean ± SD for continuous variables and number (percentage) for qualitative variables. We also assessed the correlation coefficients of serum Mg levels with several variables, including duration of ICU hospitalization, duration of mechanical ventilation, serum albumin, calcium, and potassium, as well as the score of SOFA and APACHE II. Furthermore, we performed all analyzes based on the COVID-19 infection classification (COVID-19-positive and COVID-19-negative groups). P < 0.05 was considered statistically significant.
4. Results
The final analysis was conducted on a total of 69 critically ill patients who were admitted to the ICU for a year. The findings of the study population on demographic, biochemical, and other characteristics are shown in Table 1. Mean ± SD age and BMI of individuals (53.6% females) were 52.66 ± 16.21 years and 25.93 ± 4.62 kg/m2, respectively. The mean ± SD length of ICU stay in patients was 9.43 ± 8.04 days. Out of 69 patients, 18 subjects (26.1%) died during the ICU hospitalization period, and we observed that 24 patients needed mechanical ventilation during ICU hospitalization. The mean ± SD length of mechanical ventilation in patients was 8.6 ± 6.98 days. The prevalence of COVID-19 infection among 69 patients was 39.1% (27 patients).
| Variables | Values |
|---|---|
| Gender | |
| Male | 37 (53.6) |
| Female | 24 (34.8) |
| Age | 52.56 ± 16.43 |
| Body mass index | 25.93 ± 4.62 |
| Medication history | |
| Negative | 43 (62.3) |
| Positive | 26 (37.7) |
| Mechanical ventilation | |
| Negative | 45 (65.2) |
| Positive | 24 (34.8) |
| Result of ICU hospitalization | |
| Recovery | 51 (73.9) |
| Death | 18 (26.1) |
| COVID-19 | |
| Negative | 42 (60.9) |
| Positive | 27 (39.1) |
| GCS | 13.53 ± 3.21 |
| Duration of mechanical ventilation | 8.60 ± 6.98 |
| Duration of ICU hospitalization | 9.43 ± 8.04 |
| Magnesium (mg/dL) | 2.32 ± 0.56 |
| Potassium (mmol/L) | 4.37 ± 0.88 |
| Calcium (mg/dL) | 8.90 ± 0.72 |
| Aspartate aminotransferase | 51.84 ± 39.57 |
| Alanine transaminase | 42.62 ± 25.82 |
| Alkaline phosphatase | 230.62 ± 164.68 |
Demographic, Biochemical, and Other Characteristics of Critically Ill Patients a
The results of comparing the differences between the mean Mg level of the two groups (patients with mortality or comorbidities compared to patients without mortality or comorbidities) are indicated in Table 2. Our findings demonstrated no difference in serum Mg level between patients in mortality and non-mortality groups. Moreover, no difference was found in Mg levels between patients based on comorbidity status (including sepsis and liver dysfunction). However, patients in the kidney failure group had significantly higher serum Mg than those in the non-kidney failure group (P < 0.05). No significant difference was found in the mean Mg level between patients with and without mechanical ventilation.
| Variables | Serum Mg | |
|---|---|---|
| Mean ± SD | P-Value | |
| Mortality | 0.886 | |
| Negative | 2.32 ± 0.55 | |
| Positive | 2.30 ± 0.58 | |
| Sepsis | 0.067 | |
| Negative | 2.26 ± 0.50 | |
| Positive | 2.60 ± 0.76 | |
| Liver dysfunction | 0.355 | |
| Negative | 2.29 ± 0.53 | |
| Positive | 2.45 ± 0.69 | |
| Renal failure | 0.042 | |
| Negative | 2.24 ± 0.52 | |
| Positive | 2.58 ± 0.62 | |
| Mechanical ventilation | 0.886 | |
| Negative | 2.32 ± 0.55 | |
| Positive | 2.30 ± 0.58 | |
Comparison of Mean Serum Mg Between Patients with and Without Mortality or Comorbidities a
In Table 3, the difference in Mg levels between the groups with and without mortality or comorbidities was compared based on COVID-19 infection classification (COVID-19-positive and COVID-19-negative). The results revealed no significant difference in serum Mg level between the two groups (with and without mortality or comorbidities) based on COVID-19 infection.
| Variables | COVID-19-Negative | COVID-19-Positive | ||
|---|---|---|---|---|
| Mean ± SD | P-Value | Mean ± SD | P-Value | |
| Mortality | 0.410 | 0.789 | ||
| Negative | 2.30 ± 0.53 | 2.30 ± 0.52 | ||
| Positive | 2.49 ± 0.91 | 2.25 ± 0.24 | ||
| Sepsis | ||||
| Negative | 2.26 ± 0.54 | 0.111 | 2.27 ± 0.45 | 0.602 |
| Positive | 2.63 ± 0.84 | 2.45 ± 0.21 | ||
| Liver dysfunction | 0.343 | 0.849 | ||
| Negative | 2.34 ± 0.53 | 2.22 ± 0.52 | ||
| Positive | 2.55 ± 0.77 | 2.16 ± 0.15 | ||
| Renal failure | 0.211 | 0.119 | ||
| Negative | 2.31 ± 0.54 | 2.15 ± 0.50 | ||
| Positive | 2.58 ± 0.71 | 2.57 ± 0.30 | ||
| Mechanical ventilation | 0.605 | 0.991 | ||
| Negative | 2.36 ± 0.52 | 2.22 ± 0.65 | ||
| Positive | 2.48 ± 0.87 | 2.21 ± 0.037 | ||
Comparison of Serum Mg Means Between Patients with and Without Mortality or Comorbidities Based on COVID-19 Infection Categories a
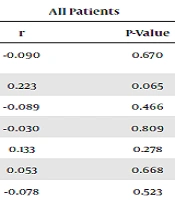
Table 4 shows the correlation coefficients between hypomagnesemia and some characteristics of patients. Our results suggested no significant correlation coefficient between hypomagnesemia and the length of mechanical ventilation and calcium and potassium serum levels. In addition, hypomagnesemia was not significantly correlated with the score of SOFA and APACHE. However, a marginal correlation was found between hypomagnesemia and the length of ICU hospitalization. Based on the COVID-19 infection classification, there was a positive correlation between hypomagnesemia and the length of ICU hospitalization in patients without COVID-19 (P < 0.05).
| Variables | All Patients | COVID-19-Negative | COVID-19-Positive | |||
|---|---|---|---|---|---|---|
| r | P-Value | r | P-Value | r | P-Value | |
| Times required mechanical ventilation | -0.090 | 0.670 | -0.282 | 0.499 | 0.132 | 0.614 |
| Duration of ICU hospitalization | 0.223 | 0.065 | 0.615 | < 0.001 | -0.097 | 0.631 |
| Albumin | -0.089 | 0.466 | -0.070 | 0.660 | -0.190 | 0.342 |
| Calcium | -0.030 | 0.809 | -0.082 | 0.607 | 0.100 | 0.618 |
| Potassium | 0.133 | 0.278 | 0.090 | 0.572 | 0.114 | 0.572 |
| SOFA | 0.053 | 0.668 | -0.018 | 0.911 | 0.219 | 0.273 |
| APACHE | -0.078 | 0.523 | -0.090 | 0.569 | -0.020 | 0.921 |
Findings on the Correlation Coefficients Between Hypomagnesemia and Some Characteristics of Patients
5. Discussion
In the current study, we assessed the difference in the serum Mg of critically ill patients based on mortality or comorbidity status. Our findings revealed no difference in the Mg levels of patients based on mortality status. Furthermore, the mean Mg level in patients with sepsis, liver dysfunction, or more extended ICU hospitalization and mechanical ventilation was not different compared to those without sepsis, liver dysfunction, or shorter ICU hospitalization and mechanical ventilation. Participants with renal failure had higher mean Mg levels than those without renal failure. Finally, the results of the stratified analysis showed no difference in Mg levels based on COVID-19 infection status except for one case in whom hypomagnesemia was positively correlated with a longer ICU hospitalization in patients without COVID-19.
The findings of the current study are comparable with the results of several investigations that previously assessed the plasma Mg status in critically ill patients admitted to the ICU. Contrary to our results, a review study indicated that patients with hypomagnesemia are more prone to increased mortality risk (7). Based on this systematic review, a 1.85-fold increment was observed in the mortality risk in patients with hypomagnesemia compared to those with a normal Mg. However, similar to our findings, they did not reveal a significant correlation between hypomagnesemia and other ancillary outcomes of ICU patients, including mechanical ventilation and ICU stay length (7). Moreover, in a systematic review and meta-analysis, Upala et al. reviewed the findings of published observational studies investigating the relationship between Mg level and the risk of mortality in individuals admitted to the ICU. Contrary to our results, they also showed a significant relationship between hypomagnesemia and the risk of mortality (a 1.9-fold increment risk of mortality) in comparison with critically ill patients with a normal Mg level (19). They reported that patients with Mg deficiency needed mechanical ventilation more frequently (RR = 1.65) and longer ICU stay length (with a mean difference of 4.1 days) than those with normomagnesemia (19). However, a study conducted on Japanese maintenance hemodialysis patients reported that although hypomagnesemia was related to malnutrition in these patients, it could not be an independent risk factor for increased risk of all-cause or cardiovascular mortality in maintenance hemodialysis patients (20). Contrary to the results of the present study, several previous studies showed that hypomagnesemia was commonly linked to a higher risk of some comorbidities, such as abdominal aortic aneurysm surgery, diabetes mellitus, blood hypertension, and sepsis. Furthermore, it has been shown that patients with hypomagnesemia were more prone to longer mechanical ventilation and ICU stay (11-16).
To the best of our knowledge, this study is the first research that has examined the risk of hypomagnesemia in COVID-19 patients admitted to the ICU based on the mortality or comorbidity status. Our results showed that the prevalence of Mg deficiency or hypomagnesemia did not rise in COVID-19 patients compared to patients without COVID-19. Faa et al. assessed the possible relationship between Mg deficiency, COVID-19, and respiratory tract and lung disorders (21). They suggested compelling reasons that hypomagnesemia can predispose individuals to COVID-19 ending with severe lung disease, often fatal (21). In other words, hypomagnesemia in individuals with COVID-19 may aggravate respiratory problems and other complications (21). The difference in the prevalence of hypomagnesemia in individuals with COVID-19 infection compared to non-infected individuals has not yet been studied in detail, and the results of our study did not show a significant finding in this regard. However, some reports suggested that in COVID-19 patients with micronutrients deficiency, such as hypomagnesemia and vitamin D deficiency, the risk of several clinical disorders, such as immune system dysfunction, increased production of cytokines and oxidative stress markers, and disseminated intravascular coagulation, may augment (22). The occurrence of the above-mentioned clinical complications can cause more damage to the pulmonary system in individuals with COVID-19 and threaten their lives. Therefore, further studies on the potential risk of Mg deficiency in different populations are recommended. In addition, Mg supplementation in the general population with special consideration can be considered during the COVID-19 pandemic (22).
We did not find any difference in the mean Mg of patients with coronavirus compared to those without coronavirus admitted to the ICU based on mortality or comorbidities status. However, it seems that various degrees of hypomagnesemia can be observed in ICU hospitalized patients. It is suggested that various organic defects, including gastrointestinal disorders or renal loss of Mg, can cause hypomagnesemia in critically ill ICU patients (23). It has been reported that the loss of gastrointestinal fluids is a common pathological condition in hospitalized patients, which may lead to the gastrointestinal loss of Mg. Indeed, various gastrointestinal disorders, including vomit, nasogastric suction, diarrhea, inflammatory bowel disease, enteritis, intestinal and biliary fistulas, pancreatitis, and intestinal surgery resections, might lead to Mg depletion and increased risk of hypomagnesemia (23). Moreover, renal loss of Mg is expected in patients in the ICU that mostly have several intravenous medications and are prone to renal medicine elimination capacity disorder, which can potentially cause urinary wasting of Mg and increased risk of hypomagnesemia. Abnormal metabolic conditions, such as diabetic ketoacidosis, alcoholism, and starvation, may lead to renal Mg wasting (23, 24).
This is the first study in Iran that investigated the Mg levels in patients with COVID-19 compared to those without COVID-19 admitted to the ICU based on mortality or comorbidities status, as well as the need for mechanical ventilation and length of stay in the ICU. However, this study had some limitations. The lack of significance in some results of our study can be partly due to the small sample size and poor power of the current study. Furthermore, this was a cross-sectional study that cannot determine causality with certainty, and therefore, we should interpret the findings with caution. Finally, we did not have information on the nutritional intake of patients and medication use, such as diuretics related to hypomagnesemia.
5.1. Conclusions
In conclusion, the current study suggested no difference in the Mg levels of patients based on mortality status. Moreover, serum Mg in patients with sepsis, liver dysfunction, or more extended ICU hospitalization and mechanical ventilation was not different from those without sepsis, liver dysfunction, or shorter ICU hospitalization and mechanical ventilation. However, patients with kidney failure had higher serum Mg than those without kidney failure. Finally, the results of the stratified analysis demonstrated no difference in Mg levels based on COVID-19 infection status. Additional studies are recommended to assess the possible relationship between hypomagnesemia and the risk of mortality and morbidity.