1. Context
The current pandemic of COVID-19 affected more than 265 800 310 million and has killed 5 266 120 million people worldwide (1). Mortality is most related to severe pneumonia caused by SARS-CoV-2 in the form of a cytokine storm. The other causes of mortality among these patients are secondary bacterial and fungal infections (2-4). Mucormycosis gained much attention in the ongoing COVID-19 pandemic (2, 5, 6). A rise in the frequency of COVID-19-associated mucormycosis (CAM) occurred in 2020 and 2021 (2, 5, 7, 8).
Mucormycosis is a severe, life-threatening, and angioinvasive disease caused by a group of molds identified as mucormycetes (2, 9). These fungi are found all over the environment, including indoor, outdoor, air filters, decaying organic materials, and on organic substrates, such as decaying fruit and vegetable matter, crop debris, bread, compost piles, animal excreta, and soil (9-12). People are exposed to microscopic spores of these fungi on a daily basis. Thus, it is probably impossible to avoid contact with mucormycetes. These fungi do not harm most healthy people (7, 9).
Spores may enter the body via the respiratory system, damaged skin, intravascular catheters, or ingestion (10). In immunocompromised patients, the main route of infection seems to be through inhalation of sporangiospores causing pulmonary infection (13). Classification of mucormycosis is based on the anatomic location, which is the route of entry of the organism into the body. Mucormycosis can be classified as rhino-orbito-cerebral mucormycosis (ROCM), pulmonary, cutaneous, gastrointestinal, and disseminated forms. Among these, ROCM is the most common type comprising about half of all mucormycosis cases. Overall, the mortality rate of ROCM has been reported to be between 54% and 75% (2, 9). Rhino-orbito-cerebral mucormycosis and pulmonary mucormycosis are mostly observed in patients with uncontrolled diabetes mellitus (DM) or immunosuppressive conditions (2, 5, 7, 9). Pulmonary mucormycosis may develop most commonly in patients with profound neutropenia and graft-versus-host disease (GVHD) (14).
Although awareness of the disease has increased, disease-associated morbidity and mortality are still high, as patients seek medical attention late in the disease process and given the low affordability of therapy (7, 8).
In this review, we aimed to provide evidence-based, comprehensive comments on the prevention, diagnosis, and management of CAM cases.
2. Methods
In the middle of 2021, with an increase in the prevalence of mucormycosis during a fifth wave, driven by the Delta variant in Iran, the Scientific Committee for the Management of Mucormycosis with the participation of medical experts was organized by the Ministry of Health and Medical Education of Iran/Vice-Chancellor’s Office in Treatment Affairs. The main purpose of this committee was to make up-to-date scientific decisions to unify the prevention and management of CAM between healthcare providers and medical centers.
2.1. Committee Composition
The committee consisted of different related specialties in infectious diseases, medical mycology, oculofacial plastic surgery, otorhinolaryngology, pharmacotherapy, ophthalmology, and emergency and internal medicine. The committee had 3 panels (i.e., prevention, diagnosis, treatment); each panel was composed of at least 5 members based on their expertise and specialty. The committee made decisions that were approved by half plus one of the members of each panel.
2.2. Evidences
Panel members reviewed the related topics and available evidence (literature review) and then summarized the findings for each section. Primary drafts were shared among panel members for commentary and discussed on at least 3 occasions in virtual meetings. Feedback from 3 external experts was obtained and used to modify the document. When strong evidence was not available to approve some decisions, the issues were voted on by the main committee. After 3 virtual or in-person meetings of the main committee, the decisions were approved and included in national guidelines.
3. Risk Factors
Mucormycosis typically affects patients with the immunocompromised state in a wide range of ages, from pre-term neonates to old-aged people (15). Rarely, mucormycosis can occur in apparently healthy people (16). Host factors play an important role in disease pathogenesis (9, 17-30).
Proposed risk factors for mucormycosis:
- Undiagnosed or poorly controlled DM
- Diabetic ketoacidosis
- Hematological and solid malignancies
- Transplantation (hematopoietic and solid organs)
- Neutropenia
- Skin damage (laceration, scratch, burn, penetration)
- Malnutrition
- High dose corticosteroid therapy (especially in diabetic patients)
- Intravenous drug abuse
- Iron overload with or without the use of deferoxamine
4. Prevention
It has been shown that various factors may predispose patients with COVID-19 to mucormycosis (20, 31-33). Noteworthy, some patients with COVID-19 and no history of DM may develop hyperglycemia following the infection (34). Fifteen percent of patients hospitalized due to COVID-19 develop new DM, and many patients develop stress-induced hyperglycemia (35). Recommendations for the prevention of CAM (20, 36, 37):
- Blood glucose levels should be monitored in all COVID-19 patients, especially hospitalized patients.
- If hyperglycemia was detected (with or without a history of DM) and no HbA1c had been measured in the past 3 months, HbA1c must be measured.
- Strict control of blood glucose (with the aim of maintaining blood glucose less than 180 mg/dL) in patients with hyperglycemia with or without a previous history of DM.
- If indicated, use appropriate doses of a corticosteroid and interleukin-6 antagonists in patients with COVID-19 (in DM patients and immunocompromised hosts with caution and the lowest possible dose).
Recommendations during and after treatment of COVID-19 with corticosteroids and immunomodulatory agents:
- Early management of hyperglycemia (if blood glucose exceeds 180 mg/dL) is essential (9, 38).
- Some patients have no history of DM and develop hyperglycemia during hospitalization. It is recommended to try controlling the hyperglycemic state during hospitalization for these patients. These patients should be referred to DM clinics when discharged (9).
- In patients with pre-existing DM, it is necessary to monitor and control the blood glucose during hospitalization and after discharge (38).
- In patients with no evidence of hyperglycemia during hospitalization but are being treated with corticosteroids, fasting blood glucose should be checked within 1 month of discharge (39).
- COVID-19 patients that have been treated with tocilizumab, corticosteroids, or have a history of DM or develop hyperglycemia during COVID-19 should be asked about visual disturbances, pain around the eyes, headache, facial pain, bleeding, nasal discharge, and numbness of the cheeks during or after COVID-19 course (40, 41). In case of manifesting any of the aforementioned warning signs, the patients should undergo a thorough examination by an expert (42, 43).
- Some anecdotal suggestions state to maintain good hygiene and avoid contact with soil, fertilizers, and spoiled materials, while patients are under treatment with immunosuppressive agents.
- Ophthalmic, nasal, and oral examinations should be regularly performed in susceptible patients, including patients with severe COVID-19, patients under mechanical ventilation, patients with current or past use of corticosteroids, or patients with a hyperglycemic state.
5. Diagnosis
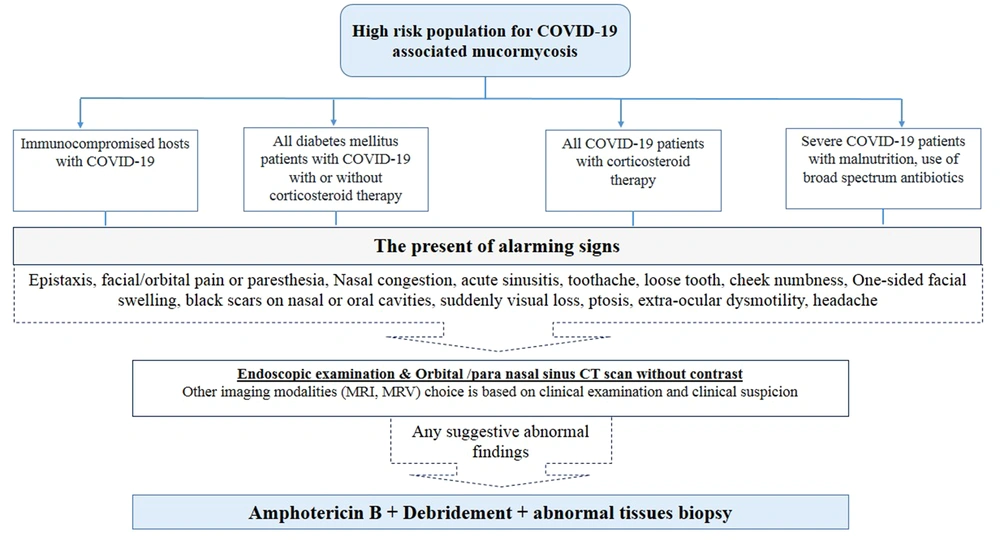
Early diagnosis of CAM is of utmost importance since it may improve outcome. Rhino-orbito-cerebral mucormycosis is the most common type observed during the current pandemic. Early treatment is essential to prevent the progression of the disease to higher stages with a poor prognosis. Therefore, to save lives and provide better care, diagnosis is an essential step for planning intensive early treatment to stop and prevent further vascular thrombosis, tissue necrosis, and loss of critical tissues of the orbit and brain (9, 18, 44-49). Diagnosis of CAM needs to network of physicians, pathologists, and mycologists (50). Upon suspicion of a CAM case, good communication and close collaboration between clinicians and the microbiology laboratory is essential to ensure that all steps of the diagnostic procedure are being taken properly. COVID-19-associated mucormycosis diagnosis is based on a combination of clinical manifestations, compatible imaging findings, and laboratory findings (Figure 1) (5, 9, 18-20).
5.1. Clinical Diagnosis
The initial features of ROCM start from nasal and paranasal sinus mucosa after spores enter through mucosa by inspiration. The infectious process can extend to nearby structures, including the brain and orbits (44-46, 48). The clinical similarities between invasive aspergillosis and mucormycosis, on the one hand, and the different treatment and prognosis of the 2 diseases, on the other, increase the importance of accurate diagnosis and differentiation of these agents (51). Early diagnosis and timely treatment of infection are essential to improve the patients’ survival before the vascular invasion, necrosis, and the spread of infection (44-46). The route of orbital extension and intra-cranial cavity are usually affected by direct fungal elements’ invasion from the sinus to the retro-orbital space and through the nasolacrimal drainage system. The angioinvasive fungus causes vascular thrombosis, ischemia, and necrosis. Disease progression is usually rapid, especially if it is not adequately managed. Necrosis over the mucosa appears as a black lesion called Eschar. In the early stages, the infected tissue may appear normal in endoscopic and direct examination (44-46). Symptoms may include nose bleed, nasal congestion, facial pain, facial hypoesthesia, headache, blurred vision, double vision, deep or periorbital pain, numbness in the nose or palate, dark nasal discharge, swelling of the face, and fever (47, 48). As the infection spreads to the orbit, ptosis and proptosis decrease muscle movement outside the eye, and visual disturbances may occur. Black necrotic lesions in the hard palate or the turbinates of the nose and septum and necrotic discharge from the eye are useful diagnostic signs (2, 45, 46).
- It is recommended that ophthalmology and otorhinolaryngology examinations be performed for high-risk patients at the time of discharge and then 2 weeks later.
- Computed tomography (CT) scan of the orbit and paranasal sinuses should be performed on each patient with cheek numbness/eyelid swelling/facial pain/nasal discharge/decreased vision and loose teeth.
- If there are any findings of sinus opacity or orbital involvement in imaging studies, the patient should be immediately referred to an experienced to an experienced physician in mucormycosis and multi-specialist and well-equipped referral centers.
- The first step is nasal endoscopic examination. If there are signs of purulent discharge, even if nasal mucosa seems normal, secretions should be obtained for mycological examination. The tissue biopsy for the mycological and pathological examination should be obtained if there is pale mucosa, nasal crusting, septal perforation, septal necrosis, loose teeth, or exposed palate bone (49, 50).
- In any patient suspected of orbital or cerebral involvement, magnetic resonance imaging (MRI) can evaluate the extent of infection (Figure 1).
- If rapid diagnostic methods and frozen sections are not available, debridement of grossly involved sinus and nasal tissues is advised (2, 45, 46).
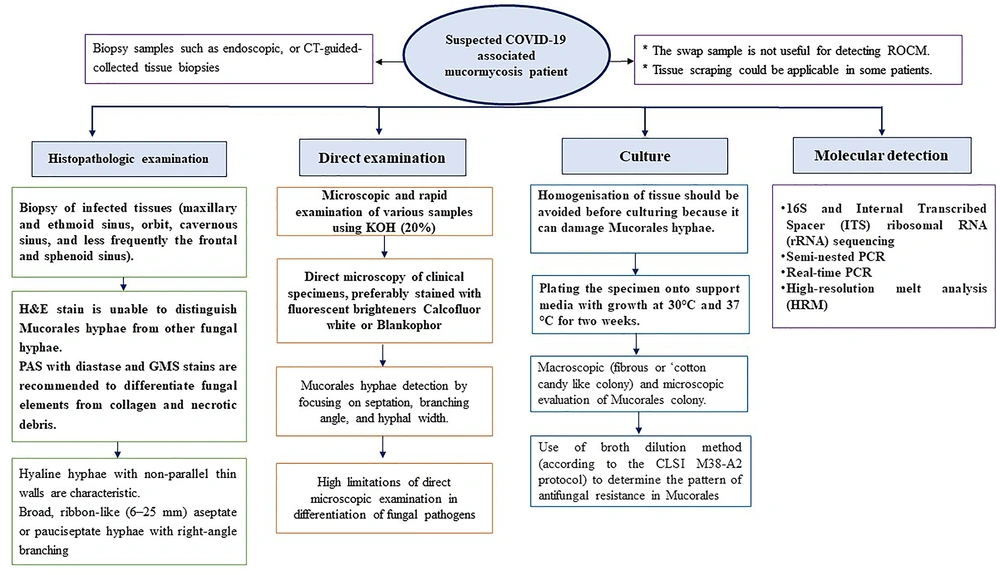
5.2. Laboratory Diagnosis
The laboratory diagnosis of CAM remains a real challenge (52). Diagnostic confirmation can be obtained by mycological (direct examination and fungal culture) and histopathology tests (53). There are no commercially available biomarkers or serological tests to detect Mucorales (48). The β-D glucan and galactomannan test does not detect antigen components of the Mucorales (9, 48). It is advised to collect biopsies of affected tissues in formalin (for a histopathology exam) and normal saline (for mycology examinations) in patients with ROCM or bronchoalveolar lavage (BAL) in patients with pulmonary mucormycosis (9, 17, 48). However, it can be challenging to take a biopsy in patients with severe thrombocytopenia or unstable systemic condition (9, 48). In these cases, upon mucormycosis suspicion, collecting serum specimens and other body fluids to detect Mucorales DNA can be attempted (9). Histopathology and mycological examinations are very important diagnostic tools since they distinguish the presence of the fungus elements (aseptate ribbon-like hyphae with right-angle branching) in the specimen from a culture contaminant and are indispensable to define whether there is blood vessel invasion (9, 48, 53). Also, these tests can furthermore reveal coinfections with other molds. Routine hematoxylin and eosin (H&E) staining may show only the cell wall with no structures inside. Stains that can help highlight the fungal wall include grocott methenamine-silver (GMS) and periodic acid-Schiff (PAS) stains, though fragmentation and necrosis of the fungal elements may cause these stains, in particular GMS, to be either faintly positive or negative. PAS gives a better visualization of the surrounding tissue compared to GMS. Direct microscopy of fresh material with potassium hydroxide (KOH) wet mounts is an inexpensive and yet invaluable method to rapidly give a presumptive diagnosis of mucormycosis. Immunohistochemistry using monoclonal antibodies against Rhizopus arrhizus has been proven useful for differentiating aspergillosis from mucormycosis (sensitivity 100% and specificity 100% for mucormycosis), particularly when cultures are negative. Identifying the definite species of the causative agent of mucormycosis to provide antifungal susceptibility tests and help for proper antifungal treatment is marginally recommended (9). Nonetheless, it should be considered that some genera, such as Cunninghamella, can be associated with an increased mortality rate in patients and have been shown to be more virulent in experimental models (9).
Most medically important Mucorales colonies grow at a temperature of 37°C within 24 - 48 hours. There is a caveat for false positive results, especially when histopathology is not available. The positive culture from a non-sterile site must be interpreted along with clinical and radiological data to establish a reliable diagnosis (49). It should be noted that fungal culture can be falsely negative in up to 50% of mucormycosis cases (44). Homogenization of tissue specimens may destroy the delicate hyphae or ongoing therapy with antifungal agents effective against Mucorales are rendering cultures negative. In these cases, attention should be paid to molecular techniques as auxiliary diagnostic tools, which can confirm the infection and identify the causal agent (9, 54). Fresh material is the preferred subject over paraffin embedded tissue because formalin damages DNA (9). Several molecular methods have been developed for the diagnosis of mucormycosis, including polymerase chain reaction (PCR)-based techniques such as nested PCR, real-time PCR, PCR followed by RFLP, PCR coupled with electrospray ionization mass spectrometry (PCR/ESI-MS), and PCR/high-resolution melt analysis (HRMA) (55). The 18S ribosomal RNA region and also other targets such as 28S rDNA, the mitochondrial gene rnl, the cytochrome b gene, or the Mucorales-specific CotH gene have been investigated for molecular detection and identification in general. All the diagnostic strategies needed to be stepwise taken for CAM diagnosis are summarized in the Figure 2.
5.3. Imaging
CT scan is the first line of imaging to quickly evaluate paranasal sinuses and orbital involvement, though, in the early stage, CT scan findings may be nonspecific (56). CT scan demonstrates sinus mucosal thickening and opacification and can often show destruction of the bony boundaries in the nose and sinuses (50). The disease progression beyond the sinuses may occur with the intact bony wall through the vascular and perineural spreading (57). Soft-tissue infiltration of deep-seated structures in CT scans is characterized by obliteration of the normal fat planes in the infratemporal fossa, pterygopalatine fossa, and pterygomaxillary fissure (57).
However, the preferred technique for diagnosis of orbital and cerebral involvement is Gadolinium-based MRI, while CT scans further help to show sinus involvement. However, MRI may be essential in the management, decision-making, and follow-up (58).
T1-weighted (T1W) and T2-weighted (T2W) images with and without fat suppression technique, including short tau inversion recovery (STIR) with gadolinium contrast and diffusion-weighted (DWI), are the basic MRI techniques required in patients with ROCM. Gadolinium-based MRI provides early detection of meningeal, intraparenchymal involvement, and also intracranial vascular occlusion (56). It has to be highlighted that the affected necrotic tissue is non-enhancing and may also show restricted diffusion on DWI. Instead, non-infracted sinusitis shows T1 post-contrast enhancement in the mucosa (57). In selected cases, magnetic resonance angiography (MRA), magnetic resonance venography (MRV), or CT angiography could help evaluate vascular invasion (44, 58).
Imaging studies are helpful for diagnosis, staging, follow-up, and delineating the progression of the disease and medical or surgical planning. There is no pathognomonic imaging sign or definite frequency for imaging (44, 55). Generally, serial MRI can be useful to diagnose progression or stop the infectious process in suspected relapse cases.
6. Treatment
Management of this potentially fatal infection is based on timely diagnosis and simultaneous use of both surgical and medical treatments (9, 48). Treatment requires a multidisciplinary team, often including infectious disease specialists, otorhinolaryngologists, orbital surgeons, ophthalmologists, endocrinologists, radiologists, pathologists, mycologists, and other disciplines such as nephrologists, neurologists, neurosurgeons, and clinical pharmacologists based on patient conditions (59).
6.1. Surgical Treatment
Surgical treatment is performed to remove necrotic tissues as much as possible, as well as to biopsy suspicious tissue (9). Debridement surgery may involve the nasal space, sinuses, and base of the skull and orbit. Complete and extensive cleaning of infected and necrotic tissues can help control the infection source and significantly increase survival (60). Accurate preoperative staging of the disease extension through imaging and pre-operation endoscopy is mandatory to indicate the surgical approach (56).
The endoscopic endonasal approach is a safe approach for debridement of the septum, nasal space, sinuses, part of the hard palate, and skull base and orbit. In the cases with the infection extension beyond these boundaries (skin or cheek involvement or more laterally seated spaces), other open approaches would be combined with endoscopic sinus surgery (ESS) to remove the lesions completely (60).
Nasal and sinus debridement includes septal resection, maxillectomy (limited or complete), ethmoidectomy, sphenoidectomy, and resection of turbines and hard palate (47). In cases of skull base involvement or clinical suspicion of skull base invasion, it is recommended to open the deep spaces of the skull base, including the pterygopalatine space, the inferior-temporal space, and the pterygoid and clinoid bone resection (47). In cases with clinical and imaging evidence of mucormycosis but nasal endoscopic findings are not diagnostic, further endoscopic examination of the basal cranial spaces is recommended in the operation room (40).
Orbital exenteration can be considered in cases when vision decreases to no light perception and loss of eye movements, along with extensive necrosis, especially in the orbital apex. Intra-orbital injection of amphotericin is a new and promising approach that may be helpful in a group of patients with orbital involvement (5, 61). Liposomal amphotericin is associated with less inflammation of the orbit after injection (40).
If imaging findings are diagnostic for osteomyelitis, the panel consensus prefers to remove all involved bones and any granulation tissues in adjacent dura and other vital neurovascular structures. In cases without invading dura by fungal elements, any normal mucosa can be saved, but the underlying involved bone should be completely removed to prevent parenchymal abscess formation. The exposed intact dura is not a proper bed for grafts or flaps, and coverage with absorbable oxidized cellulose (Surgicel) and gelatine sponge (Gelfoam) seems to be sufficient. Dura is usually very thick, and the possibility of a cerebrospinal fluid leak is slight. If there is a cerebrospinal fluid fistula, it can be reconstructed with inlay and onlay grafts. Debridement of infected brain tissue may be associated with morbidity and uncertain benefits, and fungal brain abscess can be improved with long-term medical antifungal therapy (62, 63).
6.2. Medical Treatment
Empirical amphotericin B should be promptly started in suspicious cases without wasting time on laboratory confirmation (64). Among antifungal agents, amphotericin B, posaconazole, and isavuconazole (not yet in Iranian pharmacopeia) are recommended agents for mucormycosis treatment (Table 1). Other antifungal agents have no acceptable efficacy (9, 30).
| Antifungal Agents | Dosage | Treatment Type | Adverse Effects | Monitoring | ||
|---|---|---|---|---|---|---|
| Induction | Step down | Salvage | ||||
| Liposomal amphotericin B | IV: 5 - 10 a mg/kg/d | √ | • | √ | Infusion-related reactions b Nephrotoxicity; Hypokalemia Hypomagnesaemia | BUN, serum creatinine, potassium and magnesium |
| Lipid complex amphotericin B | IV: 5 mg/kg/d | √ | • | √ | Infusion-related reactions b Nephrotoxicity; Hypokalemia Hypomagnesaemia | BUN, serum creatinine, potassium and magnesium |
| Amphotericin B deoxycholate c | IV:1 - 1.5 mg/kg/d | √ | • | √ | Infusion-related reactions d; Nephrotoxicity; Hypokalemia Hypomagnesaemia | BUN, serum creatinine, potassium and magnesium |
| Posaconazole e | Oral or IV (DR tablet or IV formulation): f 300 mg twice daily for 2 doses, then 300 mg once daily; Suspension g: 200 mg or 5 mL 4 times daily | • | √ | √ | Nausea, vomiting, diarrhea, dizziness, difficulty sleeping, thrombophlebitis in IV formulation h | Hepatic function, renal function (for IV formulation), serum electrolytes (Calcium, magnesium, potassium), drug interactions |
| Isavuconazole e | Oral or IV: 200 mg 3 times daily for 6 doses then 200 mg daily from the third day | • | √ | √ | Infusion-related reactions i Hypersensitivity reactions | Hypersensitivity reactions with initial doses, hepatic function, infusion-related reactions, drug interactions |
Recommendations on Antifungal Treatment in COVID-19-Associated Mucormycosis
Amphotericin B (1 - 10 mg/kg/d) is a standard induction treatment for mucormycosis. Higher doses are associated with greater efficacy and more side effects. Liposomal amphotericin B at 10 mg/kg/d is recommended in patients with cerebral involvement, and in other cases, a dose of 5 mg/kg/d has a sufficient and accepted effect (9). According to panel members’ recommendation, lipid formulation, especially liposomal amphotericin B, is superior to amphotericin B deoxycholate in CAM patients, and the use of amphotericin B deoxycholate is recommended when lipid formulation is not available (9).
Although there are studies on the efficacy of azoles in mucormycosis as induction treatment, due to the type of studies, at present, the panel has not recommended posaconazole and isavuconazole as first-line treatment for CAM cases (65, 66). First-line antifungal combination treatment (amphotericin B + posaconazole) may be effective in patients with cerebral involvement (62, 64, 67). There is little evidence to support combinations of polyenes and azoles or polyenes plus echinocandins (9). However, combination therapy can be rationally approached given the lack of enhanced toxicity with possible but unproven benefits. Anyhow, the evidence is insufficient to support combination therapy, and panel members recommend that if this approach is chosen, drug interactions, side effects, and treatment costs be considered. Posaconazole is recommended as a salvage regimen or step-down therapy (66-68).
Isavuconazole is a new triazole that has recently been shown to be effective as a primary or salvage treatment for mucormycosis (69). The common adverse effects of isavuconazole are nausea, vomiting, diarrhea, headaches, and fatigue (70).
The medical treatment course is a challenging decision, but the panel members generally recommended 2 - 6 weeks for amphotericin B (after the last surgical debridement) and then the same period or longer (based on the patients’ conditions in outpatient follow-up) with posaconazole or isavuconazole as a step-down therapy (59, 61, 65, 66).
Along with antifungal therapy, careful monitoring of blood urea nitrogen, serum potassium, serum creatinine, and blood glucose levels and managing patients’ comorbidities are essential (68, 71).
7. Refractory Mucormycosis
There are at least 3 main reasons to treat refractory mucormycosis: the extent and progression of the primary infection, lack of underlying condition control (such as persistent levels of serious immunodeficiency), and finally, treatment management disorders (such as drug toxicity, drug intolerance, and lack of appropriate surgical debridement) (9, 64, 72).
There is little information in the literature on the management of patients with refractory mucormycosis, but the consensus members recommend the following approach: stop or reduce the dose of corticosteroids to the lowest possible dose in the COVID-19 treatment, avoid the use of immunosuppressive agents (such as tocilizumab) in the COVID-19 management, avoid the use of broad-spectrum antibiotics in the COVID-19 management, control of hyperglycemic, perform appropriate imaging modalities according to the patient’s clinical condition to accurately determine the extent and progression of fungal lesions, design and perform debridement surgery appropriate to the patient’s clinical condition and imaging, adjust the dose of amphotericin B to a maximum of 10 mg/kg/d, and use combination salvage therapy (adding posaconazole or isavuconazole to amphotericin B).
8. Special Considerations During Treatment with Antifungal Agents
- Due to the side effects of antifungal agents, monitoring of renal and liver function tests, electrolytes, adequate hydration, and blood cell counts are recommended (9, 53).
- See Table 1 for the management of the most common side effects of amphotericin B.
- Due to the high prevalence of phlebitis in the case of intravenous posaconazole, there is a need to establish a suitable central venous line for drug delivery (66).
- Administration of intravenous posaconazole in patients with renal failure with glomerular filtration rate (GFR) less than 50 mL/min is not recommended. However, in special conditions, risks and benefits should be assessed.
- In cases with intravenous posaconazole, regular monitoring of electrolytes is recommended.
- As a potent inhibitor of cytochrome P450, consider posaconazole interactions with other drugs such as apixaban, calcium channel blockers, colchicine, cyclosporine, digoxin, domperidone, imatinib, and other drugs.
Answers to challenging questions:
- Is mucormycosis a contagious disease? The authors of this consensus could not find any conclusive evidence for the transmission of mucormycosis from human to human. Therefore, human-to-human transmission is not currently in question.
- Is mucormycosis transmitted through anesthesia machines? The panel could not find any definitive evidence for the transmission of mucormycosis through anesthesia machines.
- Should patients with mucormycosis in the COVID-19 area be isolated in the hospital or at home? There is no reason to do so. The decision on isolation is mainly based on patients’ COVID-19 conditions.
- Is antifungal prophylaxis recommended for COVID-19 patients who are at high risk for mucormycosis?According to the literature, panel members do not currently recommend the prescription of antifungal agents as prophylaxis in these patients.
- Are echinocandins effective in mucormycosis treatment? (9)The panel consensus is against the use of echinocandins in CAM patients, even as a salvage combination therapy.