1. Introduction
Coronavirus disease-2019 was reported on September 2019 in Wuhan, China, for the first time (1). Its worldwide spread caused the World Health Organization (WHO) to label it a public health emergency and international concern in January 2020, and in March 2020, it was declared a pandemic disease (2).
The most common symptoms of COVID-19 infection are fever, coughing, and dyspnea, while other symptoms include myalgia, diarrhea, sore throat, headache, fatigue, and anosmia (3, 4).
The physiopathology of this infectious disease is not fully understood (5). Potentially fatal complications could result from severe pneumonia or pulmonary edema (6).
History has proven to us that vaccines are a very effective solution to confront epidemics (7). Thus, the most important step in fighting against the COVID-19 pandemic is vaccines for acute respiratory syndrome coronavirus 2 (SARS CoV-2) (8, 9).
Most COVID-19 vaccines are designed to induce immune responses and neutralize antibodies against SARS-CoV-2 spike proteins (10).
Several vaccines, including mRNA, adenovirus vectors, protein subunits, and inactivated viruses, have proven effective in phase-III clinical trials and are now being used after emergency approval in many countries. Data from ongoing research suggest that protection might need low levels of neutralising antibodies (NAbs) and may contain other immune modulator mechanisms such as non-NAbs, T cells, and innate immune mechanisms (10).
Coronavirus disease-2019 vaccines that provoke a great amount of virus-neutralizing antibodies might be able to prevent infection. Strict clinical management and a thorough assessment of safety and immune responses are essential in vaccine trials (11).
Fourteen days after the first dose, S-binding antibodies appear and rise between 28 and 128 days later (12).
T-cell responses maximized in 14 days after the first dose, but moderately higher responses were detected 28 days after the second dose; also, tumor necrotizing factor (TNF) and interferon-γ (IFNγ) production increased by CD4+ (Cluster of Differentiation 4) T-cells at day 14 (12).
An effective, safe vaccine against COVID-19 could be the key to ending this pandemic (13). In rapid response to COVID-19 spreading in the world, some vaccines have been developed and administered on public scales; and despite their relatively quick development of them, results from several clinical trials demonstrated their remarkable efficacy and safety. To this date, more than 3 billion people have received COVID-19 vaccines and therefore reported side effects are being collected gradually (8).
The risk of vaccination complications has been evaluated in clinical trials, and their results suggest that ChAdOx1 (AstraZeneca-Oxford), ChAdOx1 (Feizer), and RNA1273 (Moderna) vaccines have been mostly well tolerated. The most commonly described side effects include local pain at the injection site, redness, swelling, and mild systemic reactions such as myalgia, headache, nausea, and fever (14).
The European Medicines Agency (EMA) approved AstraZeneca’s COVID-19 vaccine in January 2021. Although the vaccine prevented high mortality and morbidity rates from COVID-19, the occurrence of pulmonary, abdominal, and intracranial venous thromboembolic complications has increased concerns (14). Some concerns about ChAdOx1 vaccine safety led to temporary suspensions or age limit restrictions for using this particular adenovirus-based vaccine in some countries (15-17).
In four randomized controlled trials in the UK, South Africa, and Brazil, the safety and efficacy of the ChAdOx1 nCoV-19 vaccine have been evaluated and confirmed; none reported any increased incidence of events like thrombosis or thrombocytopenia (18).
Non-traumatic subarachnoid hemorrhage (SAH) results from rupturing an intracranial aneurysm in 80% of patients (19, 20). There is no sign of vascular abnormalities in about 10% of non-aneurysmal SAH. Subarachnoid haemorrhage is diagnosed by confirming of haemorrhage in subarachnoid surfaces of the brain on computed tomographic (CT) scans and by detecting an aneurysm in cerebral arteries by CT-angiography or catheter angiography (19).
Between 0.3 - 1.2 percent of patients with COVID-19 have been diagnosed with intracranial hemorrhage (including SAH). Based on Qureshi et al.’s study, patients who experienced respiratory failure, pneumonia, septic shock, and acute kidney injury were significantly higher among those who suffered from SAH and COVID-19 compared with patients with no COVID-19 (21). Also, mortality in patients with SAH and COVID-19 was remarkably higher than in patients with no COVID-19. But the risk of SAH in patients with COVID-19 wasn’t higher than in the normal population (21).
Wolf et al. suggested that exposure to the COVID-19 vaccine “AstraZeneca” might provoke the expression of anti-platelet factor (PF) antibodies, which leads to thrombocytopenia and thrombotic events (such as intracranial venous sinus thrombosis). The treatment process in those patients must consider immunological phenomena, thromboembolic features, and coagulation disorders (17).
Aneurysmal SAH patients must undergo frequent examinations, tests, and prolonged intensive care. Standard SAH treatment protocols are not properly practical in the current COVID-19 situation due to the requirement for infection control and limitations of critical care resources (22).
Considering that there are limited data on large-scale vaccinations, reliable evidence on the safety of all COVID-19 vaccines is necessary. Especially relations between COVID-19 vaccines, idiopathic thrombocytopenic purpura (ITP), and venous or arterial thromboembolic and haemorrhagic events.
Due to the lack of data on large-scale vaccinations, reliable evidence regarding the safety of COVID-19 vaccines is needed, especially regarding the relationship between COVID-19 vaccines and ITP and venous or arterial thromboembolic and hemorrhagic events (14).
According to the elevated risk of haemorrhagic events due to some COVID-19 vaccines and the highly fatal nature of cerebral haemorrhage (including SAH), it is critical to collect and investigate their relativity, risk factors, and prevention strategies. In the current study, we have reported a subarachnoid hemorrhage after receiving the first dose of the AstraZeneca vaccine in a 69-year-old man in South Khorasan, Iran.
2. Case Presentation
A 69-year-old male with no significant medical history, non-smoking, presented with headache and nausea four days after receiving the first dose of the AstraZeneca vaccine. The patient was fully conscious, and his examination showed no abnormal findings except slightly elevated blood pressure (160/100 mmHg). Primary lab data (including Complete Blood Count, coagulation tests, etc.) were in the normal range (Table 1).
| Variables | Values |
|---|---|
| CBC | |
| RBC | 4.72 × 106/mL |
| WBC | 13.3 × 103/mL |
| Platelet | 251 × 103/mL |
| Hb | 13.7 g/dL |
| Hct | 39.2% |
| BUN | 16 mg/dL |
| Creatinine | 1.0 mg/dL |
| Na | 141 mmol/L |
| K | 3.9 mmol/L |
| Ca | 9 mmol/L |
| P | 3.1 mmol/L |
| Mg | 2.19 mmol/L |
| Blood sugar | 157 mg/dL |
| CRP | Negative |
| ESR | 10 |
| PT | 15 s |
| PTT | 25 s |
| INR | 1.07 |
Abbreviations: CBC, complete blood count; RBC, red blood cells; WBC, white blood cells; Hb, hemoglobin; Hct, hematocrit; BUN, blood urea nitrogen; Na, sodium; K, potassium; Ca, calcium; P, phosphor; Mg, magnesium; CRP, C-reactive protein; ESR, estimated sedimentation rate; PT, prothrombin time; PTT, partial thromboplastin time; INR, international normalized ratio
The patient’s headache got aggravated and were unresponsive to medication, followed by vomiting, confusion, lethargy, and high blood pressure (180/100) on the second day of admission. Also, an increase in C-reactive protein (CRP) was detected on the second day.
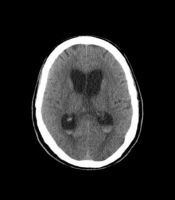
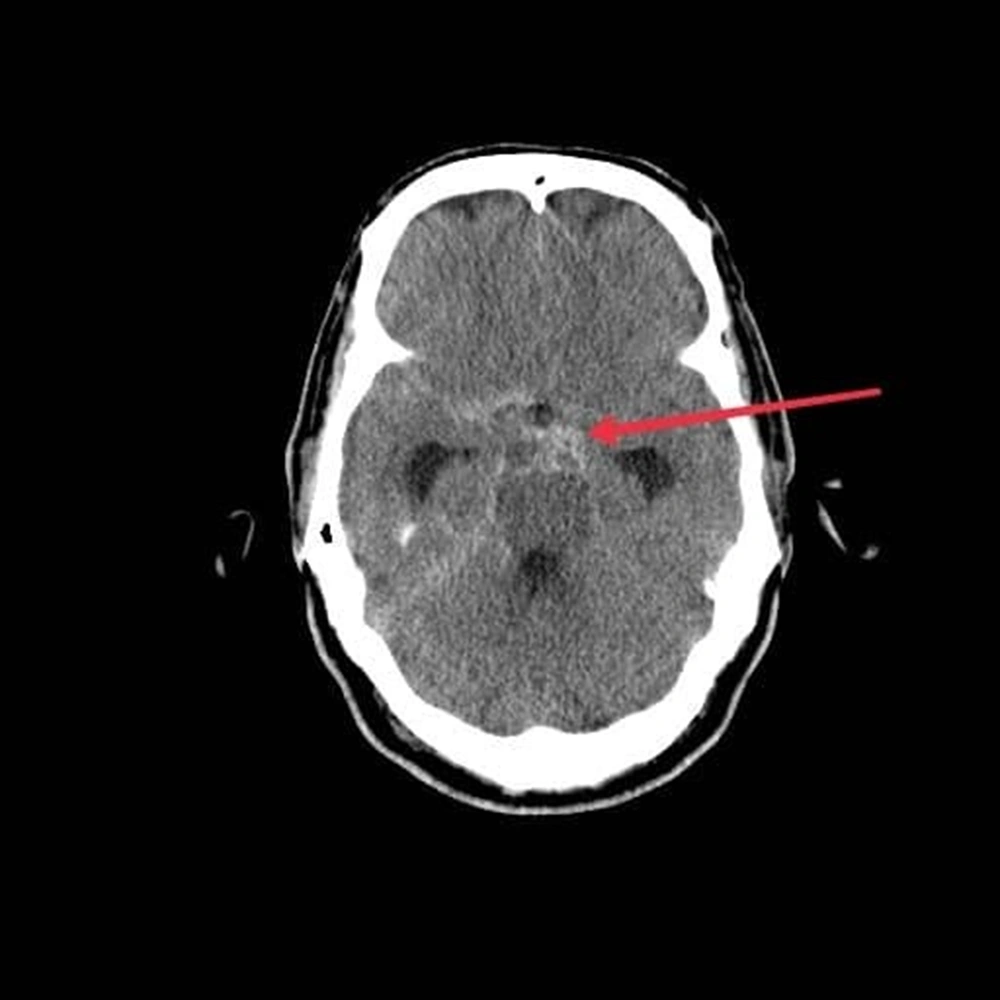
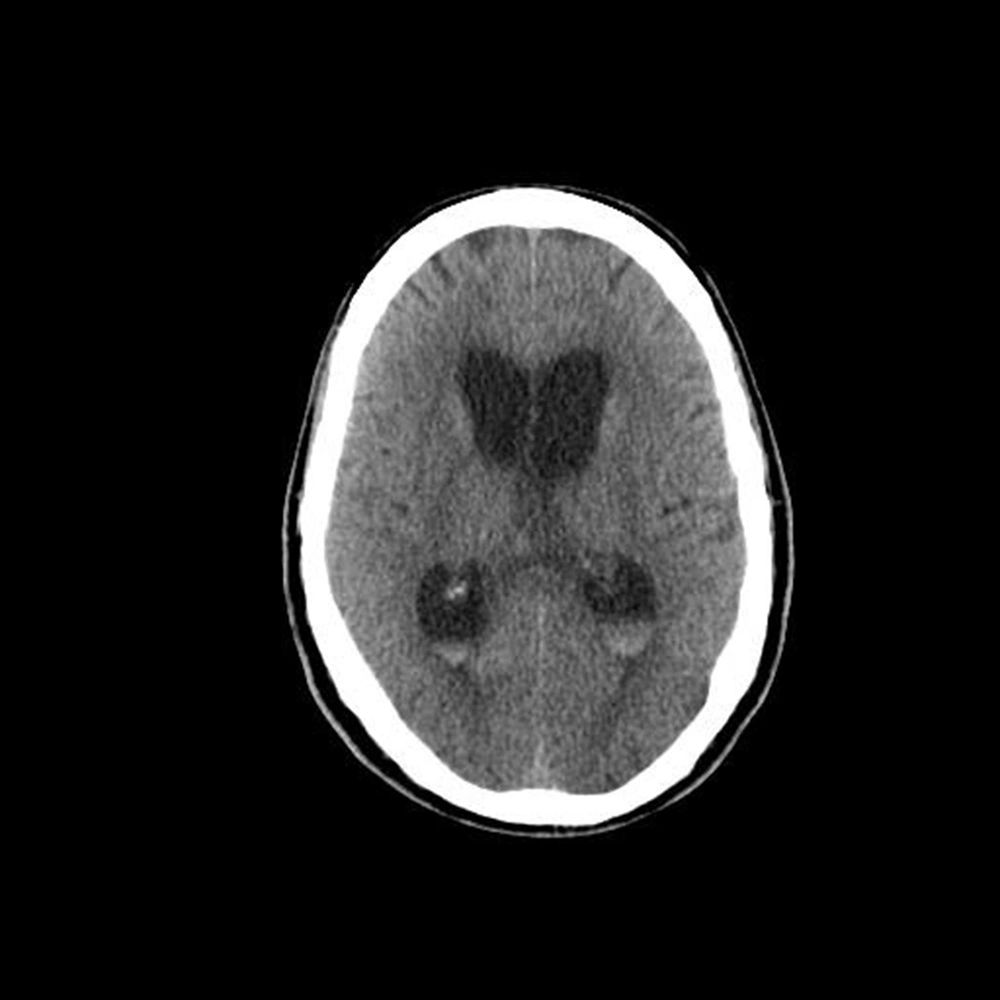
Head CT showed blood on subarachnoid surface proximate to Willis circle, associated with ventriculomegaly and periventricular edema, suggesting communicating hydrocephalus due to subarachnoid hemorrhage (Figures 1 and 2).
In order to control the raised ICP, the patient underwent an external ventricular drain (EVD) placement, leading to resolving the loss of consciousness and other symptoms. After seven days, EVD was removed, and the patient was discharged with complete recovery. Both early and delayed (after four weeks) CT angiographies showed no signs of aneurism or vascular anomalies.
3. Discussion
This study reports the occurrence of subarachnoid haemorrhage in a patient who received the first dose of the AstraZeneca vaccine against COVID-19.
Since there are rarely similar cases, it is hard to determine causes or possible relationships between the COVID-19 vaccine and SAH incidence.
There are some reports about the association between spontaneous SAH and COVID-19 infection. The results showed that spontaneous SAH is more likely to occur in the early and late course of infection with COVID-19 (23). However, we haven’t found reports of isolated SAH after receiving the COVID-19 vaccine.
Subarachnoid hemorrhage is responsible for 5 - 10% of all strokes in the United States of America. Almost 10% of non-aneurysmal subarachnoid hemorrhage patients have no vascular abnormalities; thus, no interventional treatment is considered necessary in those cases (19).
A sudden onset thunderclap headache is the hallmark symptom of aneurysmal subarachnoid hemorrhage, which grows to maximal intensity in a few seconds, usually described as the “worst headache ever” (24-26).
Hemorrhage usually occurs during normal activities but also during stressful situations (27) and may be accompanied by symptoms or signs including nuchal stiffness, nausea, vomiting, focal neurologic deficits, photophobia, and loss of consciousness (28).
Average annual SAH incidence varies among different studies and countries, from two per 100,000 population per year in China to 22.5 per 100,000 per year in Finland (29). Some of these variations might be due to differences in diagnosing rates among countries (30, 31). Subarachnoid hemorrhage incidence is more common among women; it increases with age and peaks in the 50 s (31).
In a study in Denmark and Norway on people aged between 18 and 65 who received the ChAdOx1 vaccine reports elevated rates of venous thromboembolic incidents (32).
Also, the European Medicines Agency (EMA) has received several reports of thromboembolic events, including cerebral venous and arterial thromboses, intravascular coagulation, and hemorrhagic stroke, which require further investigations (14).
Bjornstad-Tuveng et al. reported a massive intracranial hemorrhage in a previously healthy young female who had received the first dose of ChAdOx1 one week earlier (33). They found severe thrombocytopenia (37 × 109/L), PF4 antibodies, and small thrombi in the frontal lobe, transverse sinus, and pulmonary artery (33).
Simpson et al. found an association between ITP and ChAdOx1 vaccination (14). They realized that the first dose of ChAdOx1 was correlated with slightly increased risks for ITP, with suggestive evidence of risk for arterial thromboembolic and hemorrhagic incidents. They found no relations between BNT162b2 and thrombocytopenic, thromboembolic, or hemorrhagic incidents (14).
Although in the current study, we did not observe any coagulopathy or thrombocytopenia, we noticed a slight decrease in platelet count, which may be considered a relative thrombocytopenia in the first couple days. According to previous studies, some vaccines are associated with an increased risk of thrombocytopenia, ITP, and elevated levels of anti-platelet antibodies (17, 33). These pathophysiologic findings may be associated with leading to damages to cerebrovascular structures that cause incidence or worsening of other haemorrhagic manifestations such as SAH; further studies and investigations on comparable cases will be required to achieve a better understanding of the pathophysiology of this incident.
3.1. Conclusions
There are some reports of ChAdOx1 vaccine-associated thromboembolic and haemorrhagic events due to increased risk of thrombocytopenia, ITP and elevated levels of antiplatelet antibodies, however rarely, it is important to demonstrate all possible risks and complications as vaccination programs against COVID-19 have become an important part of health care in almost every country. Further studies and investigations on comparable cases will be required to achieve a better understanding about the pathophysiology of this incident. Public health authorities should notify their jurisdictions about any increased risks related to COVID-19 vaccines.