1. Background
Hepatitis E virus (HEV) is the major etiological agent of enterically-transmitted viral hepatitis (1). It belongs to Orthohepevirus, a genus within the family Hepeviridae (2). On a global estimate, about 20 million cases of infections are mainly attributable to HEV, with 34 million symptomatic cases and 70,000 deaths annually (3). Four major HEV genotypes (HEV1, HEV2, HEV3, and HEV4) have been identified to cause human infections according to phylogenetic analysis of the entire HEV genome (4). These genotypes have distinct epidemiological patterns which seem to correlate with the route of transmission, the severity of infections, mortality rates, geographic distribution, and pathogenicity levels (5, 6).
Most HEV cases are asymptomatic or cause mild clinical symptoms such as jaundice, fever, abdominal pain, and fatigue (7). Nevertheless, HEV infection can still be life-threatening, and serious clinical manifestations (chronic hepatitis, cirrhosis, and fulminant hepatitis) could occur, particularly in immunocompromised patients (8). A recent report in Lebanon has examined the prevalence of IgG anti-HEV antibodies in sera of hemodialysis patients in Tripoli and has indicated that only one pregnant woman was positive for HEV infection with a seropositivity rate of 0.22% (1/450). In addition, no significant exposure to HEV-associated risk factors has been reported (9).
Blood transfusion has been documented as a potential route of HEV transmission (10), and several cases of transfusion-acquired HEV infection have been reported in different studies worldwide (5, 10-15), highlighting the growing risk of HEV transmission via blood and blood products.
Data on HEV seroprevalence among blood donors in Lebanon are scarce, restricted to one study published in 1998, which reported a 4% HEV seropositivity rate (16).
2. Objectives
The aim of the current investigation was to get updated information on the seroprevalence of HEV among Lebanese blood donors in North Lebanon and to analyze potential risk factors associated with Hepatitis E infections. Through this approach, we aim to support the development of prevention policies in public health services in Lebanon, mainly the implementation of HEV screening for blood donations, which could reduce the associated morbidity.
3. Methods
3.1. Ethics Statement
The study was approved by the Institutional Review Board (IRB) of Nini Hospital (IRB number: IRB-F01).
Written informed consent was obtained from each participant.
3.2. Study Population
A cross-sectional study was conducted from November to December 2020, at the Blood Bank Department of Nini hospital. Blood samples were randomly collected from healthy blood donors in 1 mL ethylene-diamine-tetra-acetic acid-containing tubes (EDTA). The participants’ age was between 18 and 70 years. Subjects receiving antiviral therapy two weeks before the date of inclusion were excluded from this investigation. A standardized and detailed questionnaire was used to collect demographic characteristics and clinical information from each participant, assess potential risk factors, and get insights into the clinical history of symptomatic hepatitis.
3.3. Serological Testing
Sera samples were prepared by centrifugation at 2,000Xg for 10 min and stored at -20°C until serological testing. IgM and IgG Anti-HEV were screened using an enzyme-linked immunosorbent assay (ELISA) (DRG International, Inc. USA), according to the manufacturer’s instructions.
Cut-off values were calculated according to the following formulas: mean NC OD 450 nm/620 - 630 nm + 0.250, and mean NC OD 450 nm/620 - 630 nm + 0.350, for IgM and IgG, respectively. Anti-HEV IgM was considered negative if S/Co < 0.1, positive if > 1.2, and equivocal if the ratio was between 1.0 and 1.2. Whereas S/Co ratio < 0.9 was considered negative, positive if > 1.1, and equivocal if between 0.9 and 1.1 for anti-HEV IgG antibodies.
3.4. Statistical Analysis
Statistical analyses were performed using IBM Statistical Packages for Social Sciences (IBM SPSS, version 22.00, IBM Corp, Armonk, N.Y, USA). The association of IgM and IgG anti-HEV with potential risk factors has been examined using Chi-square or Fisher’s exact tests.
P-value < 0.05 was regarded as statistically significant.
4. Results
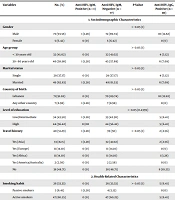
A total of 78 healthy blood donors were enrolled in the study. A standardized questionnaire was completed by each participant detailing the sociodemographic, health-related characteristics, food consumption, and lifestyle (Table 1). The median age of the research participants was 44. Table 1 shows that one blood donor had positive IgM anti-HEV antibodies corresponding to a seroprevalence rate of 1.09% (1/78). This patient was 38 years old, married, has low-to-intermediate educational level, and reported a travel history to many countries, including Syria and Turkey (Table 1). Moreover, the seroprevalence of IgG anti-HEV antibodies was reported in our study, and it reached 12.82% (10/78).
| Variables | No. (%) | Anti-HEV, IgM, Positive (n = 1) | Anti-HEV, IgM, Negative (n = 77) | P-Value | Anti-HEV, IgG, Positive (n = 10) | Anti-HEV, IgG, Negative (n = 68) | P-Value |
|---|---|---|---|---|---|---|---|
| 1. Sociodemographic Characteristics | |||||||
| Gender | > 0.05 (1) | > 0.05 (1) | |||||
| Male | 73 (93.58) | 1 (1.28) | 72 (89.74) | 10 (12.82) | 63 (80.76) | ||
| Female | 5 (6.42) | 0 (0) | 5 (6.42) | 0 (0) | 5 (6.41) | ||
| Age group | > 0.05 (1) | > 0.05 (1) | |||||
| < 30 years old | 32 (41.02) | 0 (0) | 32 (41.02) | 4 (5.12) | 28 (35.89) | ||
| 30 - 60 years old | 46 (58.98) | 1 (1.28) | 45 (57.69) | 6 (7.69) | 40 (51.28) | ||
| Marital status | > 0.05 (1) | > 0.05 (1) | |||||
| Single | 29 (37.17) | 0 (0) | 29 (37.17) | 4 (5.12) | 25 (32.05) | ||
| Married | 49 (62.83) | 1 (1.28) | 48 (61.53) | 6 (7.69) | 43 (55.12) | ||
| Country of birth | > 0.05 (1) | > 0.05 (1) | |||||
| Lebanon | 71 (91.02) | 0 (0) | 70 (89.74) | 10 (12.82) | 61 (78.20) | ||
| Any other country | 7 (8.98) | 1 (1.28) | 7 (8.98) | 0 (0) | 7 (8.97) | ||
| Level of education | > 0.05 (0.4359) | > 0.05 (0.7398) | |||||
| Low/Intermediate | 34 (43.58) | 1 (1.28) | 33 (42.30) | 5 (6.41) | 29 (37.17) | ||
| High | 44 (56.42) | 0 (00 | 44 (56.42) | 5 (6.41) | 39 (50) | ||
| Travel history | 40 (51.29) | 1 (1.28) | 39 (50) | > 0.05 (1) | 2 (2.56) | 38 (48.71) | < 0.05 (0.0448) |
| Yes (Asia) | 33 (82.5) | 1 (1.28) | 32 (41.02) | 2 (2.56) | 31 (39.74) | ||
| Yes (Europe) | 11 (14.10) | 0 (0) | 11 (14.10) | 0 (0) | 11 (14.10) | ||
| Yes (Africa) | 11 (14.10) | 0 (0) | 11 (14.10) | 1 (1.28) | 10 (12.82) | ||
| Yes (America/Australia) | 2 (2.56) | 0 (0) | 2 (2.56) | 0 (0) | 2 (2.56) | ||
| No | 38 (48.71) | 0 (0) | 38 (48.71) | 8 (10.25) | 30 (38.46) | ||
| 2. Health-Related Characteristics | |||||||
| Smoking habit | 26 (33.33) | 0 (0) | 26 (33.33) | > 0.05 (1) | 5 (6.41) | 21 (26.92) | > 0.05 (0.2873) |
| Passive smokers | 5 (6.41) | 1 (1.28) | 4 (5.12) | 0 (0) | 5 (6.41) | ||
| Active smokers | 47 (60.25) | 0 (0) | 47 (60.25) | 5 (6.41) | 42 (53.84) | ||
| Vaccinated against HEV | > 0.05 (1) | > 0.05 (0.6084) | |||||
| Yes | 10 (12.82) | 0 (0) | 10 (12.82) | 2 (2.56) | 8 (10.25) | ||
| No | 68 (87.18) | 1 (1.28) | 67 (85.89) | 8 (10.25) | 60 (76.92) | ||
| Health Complaints Prior to Six Months from Donation | |||||||
| Fever | > 0.05 (1) | > 0.05 (1) | |||||
| Yes | 10 (12.82) | 0 (0) | 10 (12.82) | 1 (1.28) | 9 (11.53) | ||
| No | 68 (87.18) | 1 (1.28) | 67 (85.89) | 9 (11.53) | 59 (75.64) | ||
| Nausea | > 0.05 (1) | > 0.05 (0.1282) | |||||
| Yes | 1 (1,28) | 0 (0) | 1 (1.28) | 1 (1.28) | 0 (0) | ||
| No | 77 (98.72) | 1 (1.28) | 76 (97.43) | 9 (11.53) | 68 (87.17) | ||
| Diarrhea | > 0.05 (1) | > 0.05 (0.4291) | |||||
| Yes | 4 (5.12) | 0 (0) | 4 (5.12) | 1 (1.28) | 3 (3.84) | ||
| No | 74 (94.88) | 1 (1.28) | 73 (93.58) | 9 (11.53) | 65 (83.33) | ||
| Stomach-ache | > 0.05 (1) | > 0.05 (0.4291) | |||||
| Yes | 4 (5.12) | 0 (0) | 4 (5.12) | 1 (1.28) | 3 (3.84) | ||
| No | 74 (94.88) | 1 (1.28) | 73 (93.58) | 9 (11.53) | 65 (83.33) | ||
| Headache | > 0.05 (1) | > 0.05 (1) | |||||
| Yes | 7 (8.97 | 0 (0) | 7 (8.97) | 1 (1.28) | 6 (7.69) | ||
| No | 71 (91.03) | 1 (1.28) | 70 (89.74) | 9 (11.53) | 62 (79.48) | ||
| Dark urine | > 0.05 (1) | > 0.05 (0.4196) | |||||
| Yes | 16 (20.51) | 0 (0) | 16 (20.51) | 3 (3.84) | 13 (16.66) | ||
| No | 62 (79.49) | 1 (1.28) | 61 (78.20) | 7 (8.97) | 55 (70.51) | ||
| Itching | > 0.05 (1) | > 0.05 (0.2414) | |||||
| Yes | 2 (2.56) | 0 (0) | 2 (2.56) | 1 (1.28) | 1 (1.28) | ||
| No | 76 (97.44) | 1 (1,28) | 75 (96.15) | 9 (11.53) | 67 (85.89) | ||
| Neurological symptoms | > 0.05 (1) | > 0.05 (1) | |||||
| Yes | 3 (3.84) | 0 (0) | 3 (3.84) | 0 (0) | 3 (3.84) | ||
| No | 75 (96.15) | 1 (1.28) | 74 (94.87) | 10 (12.82) | 65 (83.33) | ||
| Fatigue | > 0.05 (1) | > 0.05 (0.0852) | |||||
| Yes | 9 (11.53) | 0 (0) | 9 (11.53) | 3 (3.84) | 6 (7.69) | ||
| No | 69 (88.47) | 1 (1.28) | 68 (87.17) | 7 (8.97) | 62 (79.48) | ||
| Cirrhosis | > 0.05 (1) | > 0.05 (1) | |||||
| Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| No | 78 (100) | 1 (1.28) | 77 (98.71) | 10 (12.82) | 68 (87.17) | ||
| 3. Food Consumption (Last Two Weeks) | |||||||
| Food outside home consumption/month | > 0.05 (1) | > 0.05 (0.7162) | |||||
| > 1 time/month | 56 (71.79) | 1 (1.28) | 55 (70.51) | 8 (10.25) | 48 (61.53) | ||
| < 1 time/month | 22 (28.21) | 0 (0) | 22 (28.21) | 2 (2.56) | 20 (25.64) | ||
| Steak | > 0.05 (0.2692) | > 0.05 (1) | |||||
| Yes | 57 (73.07) | 0 (0) | 57 (73.07) | 7 (8.97) | 50 (64.10) | ||
| No | 21 (26.93) | 1 (1.28) | 20 (25.64) | 3 (3.84) | 18 (23.07) | ||
| Liver sausage | > 0.05 (0.2692) | > 0.05 (0.6596) | |||||
| Yes | 57 (73.07) | 0 (0) | 57 (73.07) | 8 (10.25) | 49 (62.82) | ||
| No | 21 (26.93) | 1 (1.28) | 20 (25.64) | 2 (2.56) | 9 (11.53) | ||
| Pork meat | > 0.05 (1) | > 0.05 (0.3458) | |||||
| Yes | 12 (15.38) | 0 (0) | 12 (15.38) | 0 (0) | 12 (15.38) | ||
| No | 66 (84.62) | 1 (1.28) | 65 (83.33) | 10 (12.82) | 56 (71.79) | ||
| Smoked meat | > 0.05 (1) | > 0.05 (1) | |||||
| Yes | 74 (94.87) | 1 (1.28) | 73 (93.58) | 10 (12.82) | 64 (82.05) | ||
| No | 4 (5.12) | 0 (0) | 4 (5.12) | 0 (0) | 4 (5.12) | ||
| Shellfish/seafood | > 0.05 (1) | > 0.05 (1) | |||||
| Yes | 73 (93.58) | 1 (1.28) | 72 (92.30) | 10 (12.82) | 63 (80.76) | ||
| No | 5 (6.42) | 0 (0) | 5 (6.42) | 0 (0) | 5 (6.41) | ||
| Processed meat | > 0.05 (0.2692) | > 0.05 (0.7206) | |||||
| Yes | 21 (26.92) | 1 (1.28) | 20 (25.64) | 2 (2.56) | 19 (24.35) | ||
| No | 57 (73.08) | 0 (0) | 57 (73.07) | 8 (10.25) | 49 (62.82) | ||
| Raw vegetables | > 0.05 (1) | > 0.05 (1) | |||||
| Yes | 78 (100) | 1 (1.28) | 77 (98,71) | 10 (10.82) | 68 (87.17) | ||
| No | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Raw fruits | > 0.05 (1) | > 0.05 (1) | |||||
| Yes | 73 (93.58) | 1 (1.28) | 72 (92.30) | 10 (10.82) | 63 (80.76) | ||
| No | 5 (6.42) | 0 (0) | 5 (6.42) | 0 (0) | 5 (6.41) | ||
| 4. Lifestyle-Related Characteristics | |||||||
| Water supplies | > 0.05 (0.4872) | < 0.05 (0.0448) | |||||
| Municipality taps | 40 (51.28) | 0 (0) | 40 (51.28) | 2 (2.56) | 38 (48,71) | ||
| Private wells | 38 (48.72) | 1 (1.28) | 37 (47.43) | 8 (10.25) | 30 (38.46) | ||
| Contact with contaminated water | > 0.05 (1) | > 0.05 (0.7144) | |||||
| Yes | 23 (29.48) | 0 (0) | 23 (29.48) | 2 (2.56) | 21 (26.92) | ||
| No | 55 (70.52) | 1 (1.28) | 54 (69.23) | 8 (10.25) | 47 (60.25) | ||
| Contact with domestic animals | 31 (39.75) | 0 (0) | 31 (39.75) | > 0.05 (1) | 2 (2.56) | 29 (37.17) | > 0.05 (0.2997) |
| Yes (dogs) | 16 (51.61) | 0 (0) | 16 (51.61) | 1(1.28) | 15 (19.23) | ||
| Yes (cats) | 12 (38.70) | 0 (0) | 12 (38.70) | 0 (0) | 12 (15.38) | ||
| Yes (birds/chicken/bee) | 7 (8.97) | 0 (0) | 7 (8.97) | 1 (1.28) | 6 (7.69) | ||
| No | 47 (60.25) | 1 (1.28) | 46 (58.97) | 8 (10.25) | 39 (50) | ||
a Total Number of Samples: 78.
Surprisingly, no association was found between the sociodemographic characteristics of the study participants and the anti-HEV IgM-seropositivity except for the travel history, where a significant association was reported (P = 0.044). In addition, no association was found between the health-related characteristics and anti-HEV IgM and IgG seropositivity in blood donors. Overall, the study population consisted of healthy blood donors with relatively few health complaints (Table 1). Consumption of different types of meat, pork, and shellfish showed no association with the anti-HEV IgM and IgG seropositivity (P > 0.05). Interestingly, out of all lifestyle-related characteristics, only water supplies and mainly private well usage was significantly associated with IgG anti-HEV seropositivity (P = 0.044).
5. Discussion
HEV infections cause significant morbidity and mortality worldwide, acting as a public health concern (5). These infections are mainly transmitted to humans by the fecal-oral route (water- or food -borne), or by zoonotic transmission through the consumption of raw or undercooked meat from HEV infected reservoirs, or by occupational contact with their contaminated feces (15, 17-19). An increased incidence of transfusion-transmitted HEV cases has been reported in many developed countries (19-25). However, despite the growing prevalence and the high transmission rates of HEV infections, there is no specific and efficient anti-HEV viral therapy and vaccine strategy to prevent the occurrence and improve the prognosis of HEV infection in patients. Moreover, routine screening in blood banks is still confined to developed countries, including the United Kingdom, Ireland, Netherlands, and Japan (26).
To understand the epidemiology of HEV infection in Lebanon, this study has measured the current, and previous HEV infections among blood donors in North Lebanon, through the detection of IgM and IgG anti-HEV antibodies. In addition, the most common risk factors associated with HEV infection have been identified. A seroprevalence of 1.28% (1/78), and 12.82 % (10/78) of IgM and IgG anti-HEV antibodies, respectively, was reported in our study. Other studies conducted in China and South Brazil have reported similar IgM seropositivity levels accounting for 1.13% and 1.25%, respectively (12, 13). The seroprevalence of IgM anti-HEV reported in our study was noticeably less than the seroprevalence recorded in Nepal (3.2%) and the Netherlands (8%) (5, 27). In contrast, a report from central Italy has reported lower IgM anti-HEV levels (0.6%) as compared to our findings (28).
The seroprevalence of IgG anti-HEV antibodies (12.82%) reported in our study was similar to the seroprevalence reported in Uruguay (10%) and China (13.36%) (13, 29), but remarkably lower than those reported in other countries such as Sudan (56.4%), France (54.4%), Central Italy (49%), South Africa (42.8%), Nepal (41.9%), Dutch (31%) and the Netherlands (27%) (5, 10, 11, 15, 27, 28, 30). Recently, two studies have been conducted in Lebanon to evaluate the anti-HEV IgG seropositivity in different high-risk populations such as pregnant women and hemodialysis patients (9, 31). Surprisingly, our results revealed higher anti-HEV IgG seropositivity as compared to pregnant women (0.22%), but lower than the seroprevalence reported in hemodialysis patients (21.63%). In addition, our results indicated a remarkable increase in the seroprevalence of IgG anti-HEV in blood donors compared to the prevalence (4%) reported in Lebanon in 1998, when 100 healthy blood donors were tested (16).
The difference in the reported seroprevalence over the years could be attributable to several factors, including the geographical areas examined, the diagnostic assays, the sanitation conditions, and the level of zoonotic exposure (contact with infected animals and consumption of contaminated food and water) (32). Moreover, this study has shown that travel history and water sources, mainly private wells, are important risk factors for HEV infection; since they are significantly associated with anti-HEV IgG seropositivity (P < 0.05). A potential explanation for such association is the fecal contamination of underground water compared to other sources. In addition, the contamination of underground water could also be due to the location of pipelines in the proximity of the sewer system, which increases the risk of contamination in the event of sewer leakage. In contrast, data from Sudan did not support this association, and other factors such as gender, age, locality, and animal contact were significantly associated with HEV seropositivity among the examined blood donors (11).
Furthermore, our findings showed no significant association between the seroprevalence rate of IgM and IgG anti-HEV, and the sociodemographic characteristics (except the travel history), health-related characteristics, food consumption, and lifestyle (except the water supplies) of the tested blood donors (P < 0.05). Similarly, a study in South Africa reported no significant association between the seroprevalence of IgG anti-HEV and the sociodemographic characteristics (ethnicity, gender, and place of residence) of the blood donors. However, the same study showed a statistically significant association between the seroprevalence of IgG anti-HEV and the consumption of turkey meat, and contact with rabbits or chicken (10). Nevertheless, a study conducted in the Netherlands has contradicted our findings and reported that sociodemographic characteristics, lifestyle-related factors, and food products are considered potential risk factors for HEV infection. In addition, this study has shown that contaminated water sources are significantly associated with anti-HEV IgG seropositivity (15).
5.1. Conclusions
This is the first study to investigate the seroprevalence of both IgM and IgG anti-HEV antibodies among blood donors in North Lebanon and to evaluate the associated risk factors. Our data revealed a high prevalence of IgG anti-HEV in this group, and highlighted the role of two factors in increasing the risk of HEV infection in our population. The increase in the seroprevalence of HEV among Lebanese blood donors over the years is mainly attributable to inadequate hygienic conditions, which favor the transmission of HEV by the fecal-oral route through the consumption of contaminated water and food products. In addition, the displacement of Syrian refugees to Lebanon, who lived in unfavorable sanitation conditions during the Syrian conflict, has affected the circulation rate of HEV among the Lebanese population. Thus, further epidemiological surveillance should be implemented to monitor the incidence rate of HEV under these circumstances.
Effective health strategies like blood screening before donation and health awareness regarding HEV infection are therefore required to prevent HEV circulation among blood donors. In addition, progressive improvements in sanitation conditions, provision and wide use of filtered and bottled water, and effective food safety campaigns may effectively reduce and prevent fecal-oral transmission and water-borne spread of HEV. Finally, further studies with a larger sample size and different geographical areas are needed to confirm our findings and clarify the exact transmission route of HEV among blood donors.