1. Background
Otitis externa is a frequent disorder causing pruritus, pain, edema, and erythema in the auditory canal, auricle, tympanic membrane, and middle ear (1). This condition might be infectious, inflammatory (psoriasis and eczema), or both (2). Otomycosis is defined as a superficial fungal infection, accounting for about 10% of infectious otitis externa cases (2), which might be acute, subacute, or chronic (3). This condition is more common in tropical and subtropical areas due to the hot, dusty, and humid climatic conditions (4). The prevalence of this condition in Iran ranges from 5.7% to 81% (2, 5).
Otomycosis symptoms are typically unilateral and include otalgia, hearing loss, pruritus, tinnitus, erythema, and aural discharge with debris that looks like a wet newspaper (6, 7). Sometimes fungal hyphae and spores could be observed during a clinical examination (8). The various predisposing factors to otomycosis include heat, humidity, bacterial infections, topical use of antibiotics or steroids, immunodeficiency, poor hygiene, ear surgery, frequent swimming, trauma and foreign objects, diabetes mellitus, tympanic membrane perforation, self-cleaning with cotton swabs, and seborrheic dermatitis (5, 9-12). Otomycosis tends to be caused by the genera Aspergillus (60 - 90%) and Candida (10 - 40%), with the predominance of Aspergillus niger, Aspergillus fumigatus, and Candida albicans (13).
Otomycosis accounts for about 10% of ear, nose, and throat outpatients (14). Although otomycosis mortality is very rare, the course of the disease can be exhausting due to frustrating treatment, regular follow-up, and a high recurrence rate (13). The similarity of clinical findings of otomycosis to other ear infections might result in the administration of wrong antifungals (15). Moreover, different responses to empirical antifungal therapy can lead to therapeutic failure (16).
2. Objectives
The present study aimed to investigate patients with suspicious symptoms through the examination of their demographic information, isolate etiological agents, and in vitro antifungal susceptibility patterns.
3. Methods
3.1. Collection and Identification of Samples
The samples were obtained from 170 patients with symptoms of fungal otitis externa in hospitals affiliated with Shahid Beheshti University of Medical Sciences and Tehran University of Medical Sciences, Tehran, Iran, within 2017 - 2022. The samples were collected using sterile forceps and two cotton swabs. The first swab was used for direct examination by potassium hydroxide and methylene blue. The other swab was rolled on Sabouraud Dextrose Agar (SDA, Merck, Germany) for 2 - 7 days at 30°C. The deoxyribonucleic acid of the fresh colonies was extracted using the previously described method (17, 18). The ITS1-5.8SrDNA-ITS2 of the yeast and beta-tubulin regions of the molds were amplified using the ITS1-ITS4 primers and beta-tubulin primers, respectively (19, 20). Polymerase chain reaction products were subjected to the sequence.
3.2. In vitro Antifungal Susceptibility Test
For the evaluation of the antifungal susceptibility pattern, Clinical and Laboratory Standards Institute guideline was used for yeasts (M27-A3/S4) and filamentous fungi (M38-A2) (21-23). The minimum inhibitory concentration (MIC) of miconazole, fluconazole, itraconazole, voriconazole, posaconazole, amphotericin B, and caspofungin was determined. All antifungal agents were obtained from Sigma-Aldrich, USA. The medium used for these experiments was RPMI 1640 (Sigma-Aldrich, USA) with MOPS (Sigma-Aldrich, USA).
The final ranges of drug concentrations tested were 0.064 - 64 µg/mL for fluconazole, 0.016 - 16 µg/mL for posaconazole, voriconazole, itraconazole, and amphotericin B, and 0.008 - 8 µg/mL for caspofungin. Candida suspensions were obtained from colonies grown on SDA at 35°C and were adjusted to give a final inoculum concentration of about 0.5 - 2.5 × 103 CFU/mL (24). Aspergillus suspensions were obtained after 7 days of growth on potato dextrose agar (Merck, Germany), and the cell density was adjusted to 0.4 - 2.5 × 104 CFU/mL (25).
For all drugs except amphotericin B and caspofungin, the lowest concentration of drug that caused 50% growth inhibition was regarded as MIC. Moreover, 100% inhibition of growth was MIC for amphotericin B and caspofungin.
Candida parapsilosis (ATCC 22019) was used as a quality control strain. All the tests were performed in duplicate. Since fluconazole is not commonly effective for the Aspergillus genus, this agent was not used for this species.
3.3. Statistical Analysis
Statistical analysis was performed using SPSS software (version 16.0). The chi-square test was used to test associations, and the p-value was calculated. A P-value of 0.05 or less was considered statically significant.
4. Results
The samples of 170 patients with otitis externa symptoms were collected during a 5-year period. After initial evaluations, 145 patients (85.29%) showed positive mycological findings. Table 1 shows a summary of the demographic information (e.g., gender, age, affected ear, risk factors, and clinical manifestations).
| Characteristics | No. (%) (Total = 145) |
|---|---|
| Gender | |
| Male | 81 (55.8) |
| Female | 64 (44.1) |
| Age groups (y) | |
| < 20 | 2 (1.4) |
| 20 - 29 | 14 (9.7) |
| 30 - 39 | 36 (24.8) |
| 40 - 49 | 30 (20.7) |
| 50 - 59 | 38 (26.2) |
| 60 - 69 | 15 (10.3) |
| ≥ 70 | 10 (6.8) |
| Affected ear | |
| Right | 81 (55.9) |
| Left | 60 (41.4) |
| Both | 4 (2.7) |
| Risk factor | |
| Ear manipulation | 112 (77.2) |
| Antibiotics | 82 (56.5) |
| Hearing aid | 15 (10.3) |
| Swimming | 12 (8.2) |
| Clinical manifestations | |
| Hearing loss | 134 (92.4) |
| Pruritus | 118 (81.3) |
| Otorrhea | 96 (66.2) |
| Edema | 89 (61.4) |
| Otalgia | 85 (58.6) |
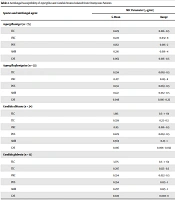
In the present study, otomycosis was observed in various occupations, with most cases observed in housewives (n = 69; 47.5%), followed by farmers (n = 24; 16.5%), employees (n = 13; 8.9%), and other occupations (n = 39; 26.8%). The seasonal distribution in patients was reported as 58 cases in winter (40%), followed by autumn (n = 45; 31%), spring (n = 22; 15%), and summer (n = 20; 13.7%). The results after sequencing showed that A. niger was the predominant species (n = 75; 51.72%), followed by A. fumigatus (n = 33; 22.75%), C. albicans (n = 24; 16.55%), and C. glabrata (n = 13; 8.96%). Table 2 summarizes the identification and antifungal susceptibility of the isolates.
| Species and Antifungal agent | MIC Parameter (µg/mL) | |
|---|---|---|
| G-Mean | Range | |
| Aspergillus niger (n = 75) | ||
| ITC | 0.229 | 0.016 - 0.5 |
| VRC | 0.223 | 0.032 - 8 |
| POS | 0.162 | 0.016 - 2 |
| AMB | 0.216 | 0.016 - 4 |
| CAS | 0.062 | 0.016 - 0.5 |
| Aspergillus fumigatus (n = 33) | ||
| ITC | 0.234 | 0.063 - 0.5 |
| VRC | 0.217 | 0.125 - 8 |
| POS | 0.134 | 0.063 - 0.5 |
| AMB | 0.122 | 0.032 - 0.5 |
| CAS | 0.046 | 0.016 - 0.25 |
| Candida albicans (n = 24) | ||
| FLC | 1.915 | 0.5 - > 64 |
| ITC | 0.339 | 0.25 - 0.5 |
| VRC | 0.115 | 0.016 - 0.5 |
| POS | 0.229 | 0.063 - 0.5 |
| AMB | 0.569 | 0.25 - 1 |
| CAS | 0.016 | 0.008 - 0.032 |
| Candida glabrata (n = 13) | ||
| FLC | 3.775 | 0.5 - > 64 |
| ITC | 0.297 | 0.125 - 0.5 |
| VRC | 0.334 | 0.032 - 0.5 |
| POS | 0.354 | 0.125 - 1 |
| AMB | 0.297 | 0.125 - 1 |
| CAS | 0.025 | 0.008 - 8 |
Abbreviations: MIC, minimum inhibitory concentration; FLC, fluconazole; ITC, itraconazole; VRC, voriconazole; POS, posaconazole; AMP, amphotericin B; CAS, caspofungin.
According to the obtained results, all tested drugs were effective against Aspergillus isolates. Caspofungin showed the highest activity against Aspergillus isolates; however, itraconazole demonstrated the lowest activity. In this study, five A. niger isolates were resistant to itraconazole (MIC: 2 µg/mL). Among A. fumigatus isolates, three isolates were resistant to amphotericin B (MIC: 8 µg/mL), and five isolates were resistant to voriconazole (MIC: 16 µg/mL).
Fluconazole showed the weakest power with a high Geometric Mean (G-Mean) against C. albicans (GM: 1.915) and C. glabrata (GM: 3.775). Caspofungin showed the most activity against C. albicans isolates (GM: 0.016) and C. glabrata isolates (GM: 0.025), respectively. Furthermore, eight C. albicans isolates were resistant to fluconazole with a MIC of 32 µg/mL. Two isolates of C. glabrata were resistant to caspofungin with a MIC of 8 µg/mL, and four isolates were resistant to itraconazole with a MIC of 2 µg/mL.
5. Discussion
Otomycosis has a global distribution with a prevalence of 4 per 1,000 individuals (26). The samples of 170 patients with otitis externa symptoms were evaluated in this study. Similar results reported by Kazemi et al. revealed that the frequency of otomycosis in a 2-year period was 92% (129 out of 140) in northwest Iran (27). However, several studies in different regions of Iran demonstrated lower frequencies of otomycosis, including Jahrom (n = 108/211; 51.1%) in the south of Iran (28), Semnan (8/70; 11.4%) in the north of Iran (1), Lorestan (15/79; 18.98%) in the west of Iran (29), Khouzestan (293/881; 32.25%) in the south of Iran (26), Yasuj (144/275; 52%) in the south of Iran (5), Rasht (43/100; 43%) in the north of Iran (30), and Isfahan (118/171; 69%) in the center of Iran (12). Based on the evidence, the prevalence of otomycosis differs in different geographical regions due to various climatic conditions (29). Therefore, the incongruity between the findings of the present study and others in Iran could be attributed to diverse geographical regions, duration of sampling, and different inclusion and exclusion criteria for patients.
Among the studied patients in this study, the prevalence was higher among those in the age range of 50 - 59 years (26.2%) but rare among adolescents ( > 20 years) and older patients (≥ 70 years). Javidnia et al. and Prasad et al. reported that otomycosis was uncommon among teenagers and older patients (28, 31). However, the results of the present study do not support those obtained in previous studies, which reported the highest prevalence of otomycosis among working groups (5, 28, 31).
Based on the present study’s results, otomycosis is more prevalent among female patients (44.1%), which is consistent with earlier reports (5, 28-30, 32, 33). However, some other studies reported higher frequency in males than in females (27, 34). The higher prevalence of otomycosis in the current study can be explained by factors, such as wearing a scarf, women’s higher tendency to visit physicians than men, and daily housework, which expose housewives to fungal spores in the dust (24, 27, 32, 35). However, wearing a head scarf was not a possible risk factor for developing otomycosis (12).
Based on previous reports, otomycosis is mostly unilateral (32). In this study, 2.7% of patients presented with the bilateral involvement of the ears, which is in line with previous studies reporting that 9%, 7%, 13.8%, and 5% of patients suffered from the simultaneous affliction of both ears, respectively (5, 11, 28, 31). A few studies reported higher rates (25% and 19.23%) of the bilateral involvement of ears (35, 36). These discrepancies might be attributed to different conditions of patients’ immune systems. Viswanatha et al. showed that bilateral otomycosis is more prevalent among immunocompromised patients than in immunocompetent patients (37).
The most common predisposing factors among the patients of the current study included ear manipulation, followed by topical antibiotic therapy, hearing aid usage, and swimming, similar to previous studies by Sabz et el. and Loh et el. in which the manipulation and self-cleaning of ears were highlighted as the most common risk factors for otomycosis (5, 38). However, the aforementioned results differ from those of other studies, which reported swimming as a major risk factor (31, 39). Furthermore, the presence of cerumen, diabetes, humid climate, hypertension, immunodeficiency, and configuration of the ear canal has been suggested as the predisposing factors of otomycosis (5, 28, 40).
In the present study, the most common symptom was hearing loss, followed by pruritus. This result is inconsistent with the results of other studies in which otalgia and pruritus were reported as the most frequent symptoms (5, 12, 15, 27, 28, 34, 41, 42). Furthermore, in two other studies, blockage of the ear (43) and otorrhea (44) were reported as the most common symptoms of otomycosis.
Based on the literature, the etiology of otomycosis is greatly divergent and has different antifungal susceptibility patterns (2, 39). In this study, out of the total 145 ears diagnosed with otomycosis, 108 and 37 ears were infected with filamentous fungi and yeast agents, respectively. A. niger was the predominant species, followed by A. fumigatus, C. albicans, and C. glabrata. Barati et al. reported that A. flavus is the most frequent etiology in otomycosis patients in central Iran (12). In opposition to the preset study’s results, Javidnia et al. reported A. tubingensis (52.7%) and A. niger (25.9%) as the most frequent isolates (28). In numerous studies, A. niger was considered to be the most prevalent etiology of otomycosis (27, 32, 33, 41, 44, 45). However, in a few studies, C. albicans was reported as the leading cause of otomycosis (29). An earlier project by García-Martos et al. showed that C. parapsilosis was the more frequent etiology of otomycosis than C. albicans (46). Some studies reported rare cases of otomycosis caused by Penicillium spp. (29) and Alternaria spp. (2, 29).
There is adequate evidence to show that azoles are the most effective agents against otomycosis without any ototoxicity (30). The results of the current study demonstrated that fluconazole, itraconazole, voriconazole, posaconazole, amphotericin B, and caspofungin were active against Aspergillus isolates, among which caspofungin and itraconazole displayed the most and the least activity against these strains, respectively. In this study, five A. niger isolates were resistant to itraconazole. Moreover, three and five A. fumigatus isolates were resistant to amphotericin B and voriconazole, respectively. In addition, caspofungin presented the highest activity against C. albicans and C. glabrata isolates; nevertheless, fluconazole showed the weakest potency. Moreover, five C. albicans isolates were considered fluconazole-resistant, and two and three C. glabrata isolates were resistant to caspofungin and itraconazole, respectively.
Szigeti et al. reported that all strains of Aspergillus showed moderate sensitivity to amphotericin B, ketoconazole, and fluconazole (47). Nemati et al. demonstrated that all A. niger isolates were sensitive to fluconazole, clotrimazole, and ketoconazole. In contrast with the results of the present study, Nemati et al. demonstrated that C. albicans isolates had the most susceptibility against fluconazole (30). Nong et al. in China reported that Aspergillus species were susceptible to itraconazole and ketoconazole, but not to fluconazole (48). The results of the aforementioned study showed that C. albicans isolates were susceptible to itraconazole, ketoconazole, fluconazole, and amphotericin B (48). Based on the evidence, the antifungal susceptibility patterns of several Aspergillus species, such as A. niger, have demonstrated variable sensitivities depending on geographical regions and various sources (49, 50).
5.1. Conclusions
Due to climatic conditions, humidity and dust, otomycosis has a high occurrence in Iran. The manipulation and self-cleaning of the ear canal with unhygienic tools were suggested as the main risk factors. Education in this regard is important to prevent this disease. To sum up, although otomycosis needs long-term antifungal therapy and recurrence is high in some cases, it is rarely life-threatening, and eardrop antifungals are usually enough to eradicate the infection.