1. Background
Coronavirus is an RNA virus belonging to the family of Coronaviridae and subfamily of Coronavirinae, first isolated in 1965 by Tyrell and Bynoe from the airways of a patient with a common cold (1). The virus is responsible for 15% of cases of common cold and pneumonia in children and young adults (2). The virus is also associated with developing and exacerbating asthma and chronic bronchitis in adults and the elderly (3). In December 2019, new cases of acute atypical respiratory disease were reported in Wuhan Province, China. Polymerase chain reaction (PCR) on a bronchoalveolar lavage (BAL) samples revealed that the cause of illness is a novel coronavirus strain, currently named SARS-CoV-2, and the resulting disease is COVID-19 infection. The disease quickly spread worldwide and became a pandemic. Early symptoms include fever, dry cough, tachypnea, and shortness of breath (4). Others include nausea, vomiting, chest pain, confusion, sore throat, sneezing, nasal congestion, decreased olfactory capacity, dyspepsia, rash, and viral conjunctivitis (5).
So far, various drugs, including antiviral drugs, antibiotics, chloroquine, hydroxychloroquine, corticosteroids, antibodies, and interferons, have been used to treat COVID-19 infection (6, 7). lopinavir antiviral drug is a viral protease inhibitor used in combination with ritonavir for treating SARS. The combination of these two drugs has been associated with a decrease in virus titer (8, 9). Ribavirin, a guanosine analog, has been used to treat infections, such as RSV, hepatitis C, and hemorrhagic fever, with promising results (10). Remdesivir antiviral drug, designed to treat the Ebola virus, has been associated with faster recovery and lower mortality than placebo (11). Metronidazole has also been suggested to be effective in COVID-19 management (12).
The usefulness of corticosteroids in the treatment of COVID-19 is controversial. In some studies, administration of corticosteroids increased the duration of hospitalization and virus excretion and had no association with a significant decline in mortality (13, 14). However, in others, the use of corticosteroids reduced the duration of hospitalization, the need for intensive critical care (ICU) hospitalization, and ventilator use, leading to an overall reduction in mortality, especially in patients requiring a ventilator (15, 16).
For instance, in a study by Fatima et al. in 2020 (17) to compare the efficacy of methylprednisolone and dexamethasone in moderate to severe patients with COVID-19 infection, there was no statistically significant difference in mortality and the need for a ventilator between the two groups. They concluded that both drugs improved the clinical and biochemical parameters in moderate-to-severe COVID-19. No consensus exists on the efficacy of diverse corticosteroids in treating COVID-19 infection.
2. Objectives
Due to the widespread prevalence of COVID-19 and the need for further studies on the role of corticosteroids, we aimed to compare the efficacy of dexamethasone and methylprednisolone in the treatment of COVID-19 infection.
3. Methods
The current retrospective cohort study reviewed the clinical records of 105 ICU COVID-19 patients treated with corticosteroids (57 patients treated with dexamethasone and 48 with methylprednisolone) at Firoozabadi hospital of Iran University of Medical Sciences, Tehran, Iran, in 2020. The required information was collected through an information form. This checklist consisted of four parts, including demographic information (age and sex), underlying diseases (hypertension, diabetes mellitus, chronic obstructive pulmonary disease, ischemic heart disease, asthma), the type of corticosteroid used, and the clinical outcome of patients (the need for a ventilator, duration of hospitalization, and mortality). Inclusion criteria included ICU COVID-19 patients over 18 years receiving dexamethasone or methylprednisolone during hospitalization. Exclusion criteria were lack of access to patients' files, history of corticosteroid use before hospitalization for any reason, mortality during the first 24 hours, or discontinuing corticosteroids due to complications.
3.1. Statistical Analysis
Data were analyzed by SPSS V.20 software (SPSS Inc. Chicago, Il, The USA). Quantitative data were presented as mean ± standard deviation (SD) and qualitative data as frequency and frequency percentage. The Kolmogorov-Smirnov test determined the normal distribution of quantitative data (age and hospitalization duration). The chi-square test explored the relationship between qualitative data. An Independent t-test (or its non-parametric counterpart, the Mann-Whitney test) evaluated any association between qualitative and quantitative variables. P < 0.05 was considered statistically significant.
3.2. Ethical Consideration
The Iran University of Medical Sciences Ethics Committee approved this study proposal (code: IR.IUMS.FMD.REC.1400.378). The researchers adhered to all the principles recommended by the Helsinki Declaration on Ethics in Research. Patients' personal and attributable information was kept confidential.
4. Results
Our subjects consisted of 105 cases. Fifty-seven (54.3%) cases were treated with dexamethasone, and 48 (45.7%) received methylprednisolone. Fifty-nine cases (56.2%) were males, and 46 (43.8%) were females. The mean age of the patients was 59.66 ± 17.09 years (16 to 98 years old). Overall, 20 (19%) patients had diabetes mellitus, 24 (22.9%) hypertension, 13 (12.4%) ischemic heart disease, six (5.7%) asthma, and five (4.8%) chronic obstructive pulmonary disease (COPD). The mean duration of hospitalization in all patients was 7.13 ± 4.06 days (2 and 21 days). Also, the mean length of hospital stay in recovered patients was 7.56 ± 3.98 days (2 and 18 days). A total of 38 (36.2%) patients needed mechanical ventilation. Finally, 57 (54.3%) patients died. Baseline comorbidities of patients are depicted in Table 1.
| Disease | Dexamethasone Group | Methylprednisolone Group | P Value (Fisher Exact Test) |
|---|---|---|---|
| Diabetes mellitus | 10 (17.5) | 10 (20.8) | 0.804 |
| Hypertension | 12 (21.1) | 12 (25) | 0.649 |
| Ischemic heart disease | 10 (17.5) | 3 (6.3) | 0.135 |
| Asthma | 5 (8.8) | 1 (2.1) | 0.216 |
| COPD | 2 (3.5) | 3 (6.3) | 0.658 |
a Values are expressed as No. (%).
The chi-square test showed no significant difference between the two groups regarding gender (P = 0.844). Also, the independent t-test showed no significant difference between the two groups regarding age (P = 0.585). Moreover, the two groups had no significant differences in underlying diseases (P values > 0.05).
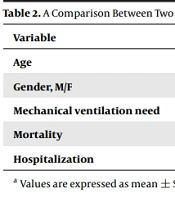
The mean duration of hospitalization was 7.21 ± 4.55 days in the dexamethasone group and 7.04 ± 4.12 days in the methylprednisolone group. The Mann-Whitney test showed no significant difference in the length of hospital stay between the two groups (P = 0.653). The Mann-Whitney test also found no significant difference in hospital stay between the two groups after the exclusion of data for patients who died (P = 0.295). Moreover, the use of invasive mechanical ventilation did not differ between the two groups using the chi-square test (P = 0.546). The two groups had no significant difference in mortality using the chi-square test (P = 0.844). A comparison between the two study groups of dexamethasone and methylprednisolone is shown in Table 2.
| Variables | Dexamethasone Group | Methylprednisolone Group | P Value (Fisher Exact Test) |
|---|---|---|---|
| Age | 58.82 ± 19.29 | 60.66 ± 14.17 | 0.585 |
| Gender, M/F | 33/24 | 26/22 | 0.844 |
| Mechanical ventilation need | 19 (33.3) | 19 (39.6) | 0.546 |
| Mortality | 30 (52.6) | 27 (56.2) | 0.844 |
| Hospitalization | 7.21 ± 4.55 | 7.04 ± 4.12 | 0.653 |
a Values are expressed as mean ± SD or No. (%).
5. Discussion
So far, various drugs, including corticosteroids, have been used to treat COVID-19 infection. The usefulness of corticosteroids in the treatment is disputed. In some studies, administration of corticosteroids was associated with an increase in the duration of hospitalization and virus excretion and had no association with a significant decline in mortality (18, 19). However, in some other studies, corticosteroids reduced the length of hospital stay, the need for ICU admission, mechanical ventilation, and overall mortality, especially in patients requiring a ventilator (20, 21).
Our study found no difference in hospital stay between the two groups. Fadel et al. (22) found that the mean duration of hospital stay was significantly lower in the methylprednisolone-treated group than in the control group. Besides, Papamanoli et al. showed that corticosteroid treatment shortened the duration of ICU stay in COVID-19 patients with severe pneumonia not requiring mechanical ventilation (23). The results of these two studies indicate the beneficial effects of corticosteroids on reducing the length of hospital stay. However, Ko et al. (16) in the USA in 2021 compared the effects of dexamethasone and methylprednisolone in COVID-19 cases and found no significant difference in the length of hospital stay in the intensive care unit between the two groups, consistent with the present study.
Contrary to the present results, Ranjbar et al. in 2021 confirmed the superior effect of methylprednisolone in reducing the length of hospital stay (24). Mora-Lujan et al. in 2021 compared high-dose methylprednisolone for three days versus low-dose dexamethasone for 10 days in severe but non-critical cases of COVID-19 infection and concluded that the length of hospital stay was significantly lower in the dexamethasone group (25). In our attempt, no significant difference was found between the two groups. However, the mentioned study examined hospitalized ICU patients with severe COVID-19-induced pupillary pneumonia, while Mora-Lujan et al. (25) evaluated non-critical cases.
The benefits of corticosteroid therapy in reducing the need for a ventilator have also been studied. In a large study, namely the RECOVERY group, dexamethasone was associated with a reduced need for aggressive mechanical ventilation (26). In addition, similar effects have been demonstrated about methylprednisolone by Fadel et al. (22) and Papamanoli et al. (23). In addition, other studies such as the current one have compared the efficacy of dexamethasone and methylprednisolone in reducing the need for mechanical ventilation. In the study by Ko et al., 45.7% in the methylprednisolone group and 43.5% in the dexamethasone group underwent mechanical ventilation without a statistically significant difference (16). The frequency of invasive ventilation was higher in the study by Ko et al. (16) than in our study. In addition, in Fatima et al. investigation in 2020 (17), the need for a ventilator was lower than in the present study, but the difference was not significant in ventilator need between the two groups.
El Mezzeoui et al. in Morocco in 2021 compared the treatment outcomes with dexamethasone or methylprednisolone in COVID-19 patients and concluded that the use of ventilators was lower significantly in the dexamethasone group (27). However, the sample size in the mentioned study was approximately five times larger than in the present study, and this difference in sample size might explain the differences in findings. On the other hand, methylprednisolone in the study by Pinzon et al. (28) and dexamethasone in the study by Mora-Lujan et al. (25) were mentioned as more effective drugs in reducing mechanical ventilation.
Finally, 52.6% in the dexamethasone group and 56.2% in the methylprednisolone group died, with no significant difference. The beneficial effects of corticosteroids in treating COVID-19 infection and the resulting reduction in mortality have also been studied. In the RECOVERY group's study, dexamethasone significantly decreased 28-day death among people receiving invasive ventilation (26). In addition, in Fadel et al. (22) and Papamanoli et al. (23), methylprednisolone had similar effects in reducing mortality compared to the control group. The results of these studies indicate corticosteroids' beneficial effect in reducing the mortality rate. The data from a meta-analysis by the World Health Organization (WHO) research group called the REACT in 2020 also showed the effect of corticosteroids in significantly reducing patients' mortality (29).
In addition, some other studies compared dexamethasone and methylprednisolone in reducing patient mortality. The prevalence of mortality in the study by Fatima et al (17). in the two groups receiving dexamethasone and methylprednisolone was reported as 17.1% and 15.3%, respectively, with no statistically significant difference. Moreover, in 2021 in Morocco, El Mezzeoui et al. (27) reported mortality of 27.91% in the dexamethasone group and 35.6% in the methylprednisolone group, inconsistent with our findings. As mentioned earlier, a much larger sample size of the above study may justify this difference. Mora-Lujan et al. (25) also reported that dexamethasone was more effective in reducing mortality. Nonetheless, in 2021 in Colombia, Pinzon et al. described methylprednisolone as a more effective drug in reducing patient mortality (28). Therefore, due to the high variation in the results of studies worldwide, it is necessary to perform meta-analyses in this field.
We suffered from several limitations. This study had a retrospective design, and there would be the risk of a recall bias. Also, documents' data might not have been completed covering all aspects of patients' information due to the pandemic issue and staff shortage. Moreover, as the disease course is not well elucidated, we could not run a randomized prospective study. Furthermore, the sample size could be larger. It is suggested to perform larger studies in different centers covering varied ethnicities to clarify the effect of different corticosteroids on mortality and morbidity.
5.1. Conclusions
Our findings indicated no significant difference in the mean duration of hospital stay, need for a ventilator, and mortality in the COVID-19 ICU patients who received either methylprednisolone or dexamethasone.