1. Background
Our current world has undergone a huge transformation in various social, economic, and public health dimensions with the spread of the infectious agent called SARS-CoV-2. Coronavirus disease 2019 (COVID-19), in addition to taking the lives of countless people around the world, has threatened the survival of patients with a variety of consequences. Many patients with this infection show reduced symptoms, but some patients experience dysfunction of the various organs, which can be traced back to the physiological pathways associated with the clinical symptoms (1). After passing through the viral replication phase, SARS-CoV-2 induces widespread inflammatory responses in the host body by acting on immunological pathways (2). At this stage, phagocytic leukocytes such as neutrophils, eosinophils, monocytes, and macrophages stimulate the inflammatory mechanism by producing free radicals, mediating nuclear factor-kappa B (NF-κB) activation, and inducing transcription of cytokine-producing genes (3, 4). The proper functioning of the immune system depends on being safe from the harmful effects of overproduction of these compounds and, thus, the presence of a sufficient level of antioxidant defense (5).
Although definitive treatment for the infectious agent SARS-CoV-2 has not been reported, the treatment process is generally followed by the administration of immunosuppressive drugs, antiviral drugs, as well as anti-inflammatory drugs. Methylprednisolone is one of the anti-inflammatory compounds used to reduce the immune response in many diseases. This corticosteroid is used in acute respiratory infections due to its anti-inflammatory effects, reduction of immune reactions, and improvement of clinical conditions (5). Studies have shown that the use of methylprednisolone in acute respiratory distress syndrome (ARDS) patients leads to a sustained decrease in inflammatory plasma cytokines and chemokines and also improves lung damage and multiple dysfunction syndromes (MODS). The use of methylprednisolone can also effectively reduce the time of mechanical ventilation and also mortality in the ICU (6). In addition, this drug has recently been used in the treatment of patients with SARS-CoV-2 infection (6).
2. Objectives
Considering the role of anti-inflammatory drugs in improving respiratory infections and considering the importance of antioxidant balance in controlling the inflammatory process, we investigated the effect of high dose methylprednisolone on clinical symptoms and antioxidant changes in patients with COVID-19.
3. Methods
The present study was performed as a non-randomized and non-blind comparative study with the ethical code IR.SBMU.NRITLD.REC.1399.227. Patients with COVID-19 (disease detection based on PCR and CT-SCAN molecular test) referred to Masih Daneshvari Hospital in Tehran from January to July 2021, who required hospitalization and were in moderate to severe disease. If they had inclusion criteria, they entered the study phase after obtaining written consent (predicted sample size: 30 patients). Inclusion criteria included evidence of new coronavirus (SARS-CoV-2) (clinical or paraclinical), saturation < 90, written consent of the study participants, age over 18 years, moderate to severe COVID-19 disease hospitalized, and in need of respiratory support. Exclusion criteria included pregnant or lactating patients, gastrointestinal bleeding, and a history of allergy to steroid drugs.
Then, in addition to standard treatment, patients received methylprednisolone at a dose of 250 mg intravenously over three days. Necessary evaluations, including arterial blood gas analysis, pulse oximetry, monitoring of patient clinical signs, and evaluation of inflammatory biomarkers, were performed. Also, receiving 10 cc of peripheral blood samples to study antioxidant changes was on the agenda at the beginning of the study, after 24 hours, and 72 hours after receiving methylprednisolone.
3.1. Statistical Analysis
In this study, quantitative variables were analyzed using the mean and standard deviation (SD), as well as qualitative variables using numbers (by mentioning percentages). To check the normality of quantitative variables, the Kolmogorov-Smirnov test was used using box diagrams. All the statistical tests used in this study were performed in two domains using SPSS 22 software, and the significance level was also considered to be 5%.
4. Results
Based on the results of this study, the population ratio was the same in patients, and according to Table 1, the average age of patients was 49.827 ± 12.803 years. This value was 52.40 ± 14.48 in the severe group of patients and 47.07 ± 10.56 in the moderate group. The numerical value of patients’ weight also showed that although the mean weight of all patients was 78.250 ± 12.438 and had no difference in the two groups significantly (P = 0.543), this numerical value was higher in patients in the severe group with 79.71 ± 15.19 kg than the average group with 76.79 ± 9.27. Due to the approximately similar height of patients in the two groups, the BMI index was higher in patients with severe coronavirus than in the average group. Table 1 shows that these results are also true for the two indicators of the length of hospital stay and duration of hospitalization. Thus, in terms of the duration of illness to hospitalization, people in the severe group with an average of 10.40 ± 4.97 days went to treatment units significantly later (P = 0.006) compared to the moderate group with an average of 6.07 ± 2.84 days. However, the total mean time to hospitalization was 8.310 ± 4.575 in all patients.
| Groups | Total | Moderate, Mean ± SD | Severe, Mean ± SD | P-Value |
|---|---|---|---|---|
| Age | 49.827 ± 12.803 | 47.07 ± 10.56 | 52.40 ± 14.48 | 0.270 |
| Weight | 78.250 ± 12.438 | 76.79 ± 9.27 | 79.71 ± 15.19 | 0.543 |
| Height | 167.071 ± 11.712 | 166.86 ± 10.85 | 167.29 ± 12.93 | 0.925 |
| BMI | 28.580 ± 4.611 | 28.59 ± 3.60 | 28.57 ± 5.53 | 0.992 |
| Hospital stays | 7.482 ± 2.429 | 6.86 ± 0.86 | 8.07 ± 3.22 | 0.769 |
| The onset of symptoms is referred to | 8.310 ± 4.575 | 6.07 ± 2.84 | 10.40 ± 4.97 | 0.006* |
Evaluation of Demographic Information of All Patients and Comparison of These Indicators Based on Two Groups of Patients with Severe and Moderate Forms of COVID-19
According to Table 2, which examines the course of changes in patients’ respiratory and clinical indices, the HR index in both groups was significantly reduced (P < 0.005). This change was significantly reduced for fever index (in patients with moderate severity) and Borg scale (in both groups) (P < 0.005). Although the course of changes in Spo2 and PaCO2 indices increased in the group of patients with a moderate form of the disease, the changes were not significant. However, the course of changes is constant in the two indicators of fever (in the group of patients with severe form) and RR. Also, urea, ALT, and bilirubin indices increased in both groups of patients and showed significant changes (P < 0.005). However, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) had a significant decreasing trend in both groups (P < 0.005). It should be noted that the two indices of creatinine (Cr) in severe patients and LDH in moderate patients were significantly reduced (P < 0.005).
| Mean ± SD | Percentiles | P-Value | |||
|---|---|---|---|---|---|
| 25th | 50th | 75th | |||
| HR | |||||
| Moderate | 0.006 | ||||
| Before | 95.2143 ± 14.159 | 83.750 | 95.500 | 108.500 | |
| After 24 h | 82.0000 ± 14.071 | 73.750 | 78.000 | 89.2500 | |
| After 72 h | 79.0000 ± 8.3757 | 72.200 | 80.500 | 85.2500 | |
| Severe | 0.004 | ||||
| Before | 107.1333 ± 26.51 | 94.000 | 110.00 | 120.000 | |
| After 24 h | 86.6000 ± 7.3949 | 80.00 | 85.000 | 93.0000 | |
| After 72 h | 82.2667 ± 5.7004 | 77.000 | 82.000 | 86.0000 | |
| RR | |||||
| Moderate | 0.078 | ||||
| Before | 17.8571 ± 0.9492 | 18.000 | 18.000 | 18.0000 | |
| After 24 h | 18.8571 ± 1.5118 | 18.000 | 18.000 | 20.0000 | |
| After 72 h | 18.5714 ± 1.2225 | 18.000 | 18.000 | 18.5000 | |
| Severe | 0.439 | ||||
| Before | 19.1333 ± 1.2459 | 18.000 | 19.000 | 20.0000 | |
| After 24 h | 18.8667 ± 1.6417 | 18.000 | 18.000 | 20.0000 | |
| After 72 h | 18.6667 ± 0.9759 | 18.000 | 18.000 | 20.0000 | |
| Fever | |||||
| Moderate | 0.017 | ||||
| Before | 36.7786 ± 0.2778 | 36.500 | 36.700 | 37.0000 | |
| After 24 h | 36.6286 ± 0.2701 | 36.500 | 36.550 | 36.9250 | |
| After 72 h | 36.5143 ± 0.1657 | 36.500 | 36.500 | 36.6000 | |
| Severe | 0.853 | ||||
| Before | 36.7929 ± 0.5239 | 36.500 | 36.650 | 36.8500 | |
| After 24 h | 36.6571 ± 0.3081 | 36.500 | 36.600 | 37.0000 | |
| After 72 h | 36.6500 ± 0.1605 | 36.500 | 36.600 | 36.7250 | |
| Borg scale | |||||
| Moderate | 0.003 | ||||
| Before | 5.3636 ± 2.06265 | 4.0000 | 5.0000 | 8.0000 | |
| After 24 h | 4.7273 ± 1.90215 | 3.0000 | 4.0000 | 7.0000 | |
| After 72 h | 3.5455 ± 1.43970 | 2.0000 | 3.0000 | 5.0000 | |
| Severe | 0.006 | ||||
| Before | 4.4545 ± 2.54416 | 3.0000 | 5.0000 | 5.0000 | |
| After 24 h | 3.3636 ± 1.68954 | 2.0000 | 3.0000 | 5.0000 | |
| After 72 h | 2.7273 ± 1.95402 | 2.0000 | 2.0000 | 5.0000 | |
| SpO2 | |||||
| Moderate | 0.502 | ||||
| Before | 94.7143 ± 1.89852 | 93.000 | 95.000 | 96.0000 | |
| After 24 h | 94.6429 ± 1.44686 | 93.000 | 94.500 | 96.0000 | |
| After 72 h | 95.2857 ± 1.85757 | 94.750 | 95.500 | 96.0000 | |
| Severe | 0.225 | ||||
| Before | 93.0667 ± 2.89005 | 90.000 | 94.000 | 96.0000 | |
| After 24 h | 94.3333 ± 1.83874 | 93.000 | 95.000 | 96.0000 | |
| After 72 h | 91.8267 ± 8.74524 | 92.000 | 94.000 | 96.0000 | |
| PaCO2 | |||||
| Moderate | 0.212 | ||||
| Before | 47.100 ± 10.5014 | 39.500 | 49.000 | 51.500 | |
| After 24 h | 48.169 ± 5.67104 | 42.900 | 47.000 | 54.500 | |
| After 72 h | 52.430 ± 13.3051 | 44.500 | 52.000 | 62.500 | |
| Severe | 0.982 | ||||
| Before | 50.357 ± 5.95751 | 46.500 | 51.000 | 53.500 | |
| After 24 h | 47.628 ± 8.72683 | 40.750 | 46.350 | 53.000 | |
| After 72 h | 50.435 ± 8.18795 | 45.750 | 51.100 | 57.650 | |
| WBC | |||||
| Moderate | 0.0397 | ||||
| Before | 7.333 ± 3.79006 | 4.5000 | 6.4000 | 10.100 | |
| After 24 h | 8.250 ± 3.38369 | 5.2650 | 8.2000 | 10.475 | |
| After 72 h | 9.293 ± 3.16487 | 6.7100 | 10.4900 | 11.500 | |
| Severe | 0.789 | ||||
| Before | 14.911 ± 22.5218 | 5.7475 | 8.7500 | 11.125 | |
| After 24 h | 9.155 ± 4.44306 | 4.9625 | 8.4700 | 12.207 | |
| After 72 h | 11.241 ± 7.45625 | 6.1625 | 8.5700 | 13.617 | |
| Urea | |||||
| Moderate | 0.004 | ||||
| Before | 30.571 ± 12.768 | 21.0000 | 24.500 | 42.250 | |
| After 24 h | 37.000 ± 15.491 | 28.5000 | 33.500 | 42.250 | |
| After 72 h | 44.428 ± 10.278 | 37.7500 | 46.000 | 53.000 | |
| Severe | 0.001 | ||||
| Before | 33.400 ± 13.589 | 28.0000 | 32.000 | 41.000 | |
| After 24 h | 42.266 ± 18.926 | 33.0000 | 37.000 | 44.000 | |
| After 72 h | 53.000 ± 28.886 | 38.0000 | 45.000 | 55.000 | |
| Cr | |||||
| Moderate | 0.066 | ||||
| Before | 1.078 ± 0.196 | 0.900 | 1.100 | 1.200 | |
| After 24 h | 1.014 ± 0.210 | 0.800 | 1.000 | 1.125 | |
| After 72 h | 0.985 ± 0.313 | 0.875 | 1.050 | 1.200 | |
| Severe | 0.001 | ||||
| Before | 1.726 ± 2.107 | 1.100 | 1.100 | 1.300 | |
| After 24 h | 1.320 ± 1.057 | 1.000 | 1.100 | 1.200 | |
| After 72 h | 1.280 ± 1.173 | 1.000 | 1.100 | 1.200 | |
| AST | |||||
| Moderate | 0.257 | ||||
| Before | 33.714 ± 11.179 | 26.750 | 32.000 | 40.250 | |
| After 24 h | 39.714 ± 10.313 | 31.500 | 37.500 | 47.500 | |
| After 72h | 43.714 ± 20.693 | 24.750 | 37.000 | 55.000 | |
| Severe | 0.138 | ||||
| Before | 37.142 ± 16.737 | 24.500 | 34.500 | 47.500 | |
| After 24 h | 36.142 ± 13.960 | 23.750 | 34.500 | 49.250 | |
| After 72h | 43.357 ± 21.168 | 25.750 | 39.500 | 50.750 | |
| ALT | |||||
| Moderate | 0.017 | ||||
| Before | 36.857 ± 17.064 | 24.000 | 35.500 | 48.250 | |
| After 24 h | 41.000 ± 14.889 | 36.000 | 40.500 | 44.750 | |
| After 72 h | 69.857 ± 49.155 | 47.000 | 56.000 | 61.750 | |
| Severe | 0.008 | ||||
| Before | 36.133 ± 23.008 | 17.000 | 32.000 | 46.000 | |
| After 24 h | 34.400 ± 15.701 | 17.000 | 33.000 | 53.000 | |
| After 72 h | 67.133 ± 42.914 | 27.000 | 65.000 | 85.000 | |
| ALP | |||||
| Moderate | 0.395 | ||||
| Before | 142.000 ± 40.876 | 102.250 | 148.000 | 173.50 | |
| After 24 h | 164.285 ± 118.781 | 97.750 | 141.500 | 153.00 | |
| After 72 h | 136.714 ± 31.699 | 105.250 | 135.500 | 164.50 | |
| Severe | 0.124 | ||||
| Before | 191.733 ± 151.732 | 93.000 | 124.000 | 251.00 | |
| After 24 h | 204.000 ± 194.579 | 113.000 | 133.000 | 210.00 | |
| After 72 h | 170.333 ± 121.917 | 111.000 | 145.000 | 153.00 | |
| Bil | |||||
| Moderate | 0.031 | ||||
| Before | 0.669 ± 0.375 | 0.400 | 0.600 | 0.900 | |
| After 24 h | 0.515 ± 0.293 | 0.300 | 0.500 | 0.650 | |
| After 72 h | 0.715 ± 0.237 | 0.550 | 0.700 | 0.900 | |
| Severe | 0.014 | ||||
| Before | 0.666 ± 0.179 | 0.500 | 0.700 | 0.800 | |
| After 24 h | 0.480 ± 0.169 | 0.400 | 0.400 | 0.600 | |
| After 72 h | 0.646 ± 0.289 | 0.400 | 0.700 | 0.900 | |
| CRP | |||||
| Moderate | 0.002 | ||||
| Before | 50.583 ± 21.334 | 41.250 | 44.000 | 60.250 | |
| After 24 h | 41.416 ± 24.254 | 23.000 | 39.000 | 49.000 | |
| After 72 h | 15.833 ± 25.711 | 3.250 | 8.500 | 14.250 | |
| Severe | < 0.001 | ||||
| Before | 54.357 ± 22.245 | 41.750 | 54.500 | 60.250 | |
| After 24 h | 71.071 ± 24.627 | 47.750 | 64.000 | 96.500 | |
| After 72 h | 24.000 ± 12.428 | 13.750 | 22.000 | 30.500 | |
| LDH | |||||
| Moderate | < 0.001 | ||||
| Before | 516.285 ± 96.497 | 448.750 | 493.000 | 595.000 | |
| After 24 h | 506.142 ± 124.445 | 419.000 | 503.500 | 559.250 | |
| After 72 h | 427.785 ± 88.105 | 373.500 | 432.000 | 460.500 | |
| Severe | 0.091 | ||||
| Before | 545.466 ± 192.425 | 392.000 | 506.000 | 749.000 | |
| After 24 h | 518.400 ± 148.267 | 375.000 | 532.000 | 627.000 | |
| After 72 h | 491.533 ± 171.835 | 375.000 | 404.000 | 659.000 | |
| ESR | |||||
| Moderate | < 0.001 | ||||
| Before | 45.153 ± 22.575 | 28.500 | 50.000 | 64.000 | |
| After 24 h | 54.461 ± 28.215 | 24.500 | 61.000 | 75.500 | |
| After 72 h | 25.615 ± 17.153 | 7.500 | 24.000 | 41.000 | |
| Severe | 0.001 | ||||
| Before | 53.000 ± 22.934 | 36.250 | 47.500 | 74.000 | |
| After24 h | 50.285 ± 21.620 | 37.500 | 49.000 | 64.500 | |
| After 72 h | 22.357 ± 12.743 | 12.750 | 20.000 | 31.500 | |
Evaluation of Changes in Clinical and Biochemical Indicators in the Two Groups of Patients with Severe and Moderate Forms of COVID-19
Examination of the antioxidant status of patients in the two groups treated with methylprednisolone showed that all antioxidants evaluated in this study underwent significant changes in both groups of patients (P < 0.005) (Table 3). Changes in the immunological factors of the patients showed that TNF-α significantly decreased in patients of both severe and moderate groups with COVID-19 after five days (P < 0.001). IL-10 increased significantly in both groups after five days (P < 0.001). Also, ferritin significantly decreased in both groups of severe and moderate patients after five days (P < 0.001 in the severe group and P = 0.002 in the moderate group) (Table 4).
| Mean ± SD | Percentiles | P-Value | |||
|---|---|---|---|---|---|
| 25th | 50th | 75th | |||
| SOD | |||||
| Moderate | 0.023 | ||||
| Day 1 | 2.128 ± 0.140 | 1.970 | 2.195 | 2.242 | |
| Day 2 | 2.419 ± 0.293 | 2.200 | 2.270 | 2.762 | |
| Day 5 | 2.236 ± 0.360 | 2.087 | 2.305 | 2.495 | |
| Severe | < 0.001 | ||||
| Day 1 | 1.563 ± 0.189 | 1.430 | 1.550 | 1.600 | |
| Day 2 | 1.962 ± 0.091 | 1.900 | 1.930 | 2.010 | |
| Day 5 | 9.140 ± 27.625 | 1.920 | 1.980 | 2.110 | |
| GPX | |||||
| Moderate | 0.708 | ||||
| Day 1 | 19.717 ± 0.404 | 19.470 | 19.820 | 20.087 | |
| Day 2 | 19.814 ± 0.345 | 19.717 | 19.830 | 19.947 | |
| Day 5 | 19.953 ± 0.431 | 19.807 | 19.930 | 20.185 | |
| Severe | 0.038 | ||||
| Day 1 | 18.802 ± 0.630 | 18.170 | 18.880 | 19.230 | |
| Day 2 | 18.943 ± 0.750 | 18.200 | 18.970 | 19.500 | |
| Day 5 | 19.082 ± 0.645 | 18.410 | 19.270 | 19.660 | |
| GST | |||||
| Moderate | 0.003 | ||||
| Day 1 | 0.882 ± 0.036 | 0.865 | 0.890 | 0.910 | |
| Day 2 | 0.902 ± 0.053 | 0.855 | 0.895 | 0.952 | |
| Day | 0.939 ± 0.035 | 0.907 | 0.940 | 0.970 | |
| Severe | < 0.001 | ||||
| Day 1 | 0.567 ± 0.106 | 0.520 | 0.540 | 0.570 | |
| Day 2 | 0.628 ± 0.098 | 0.580 | 0.600 | 0.640 | |
| Day 5 | 0.897 ± 0.037 | 0.870 | 0.900 | 0.920 | |
| FRAP | |||||
| Moderate | 0.330 | ||||
| Day 1 | 616.73 ± 8.538 | 613.750 | 618.70 | 621.050 | |
| Day 2 | 618.10 ± 6.387 | 614.602 | 619.80 | 622.300 | |
| Day 5 | 618.85 ± 3.463 | 616.100 | 619.05 | 621.117 | |
| Severe | < 0.001 | ||||
| Day 1 | 598.36 ± 9.175 | 590.940 | 598.80 | 603.480 | |
| Day 2 | 617.72 ± 3.763 | 617.800 | 619.24 | 620.000 | |
| Day 5 | 617.98 ± 3.222 | 617.090 | 619.44 | 620.090 | |
| ALB | |||||
| Moderate | < 0.001 | ||||
| Day 1 | 4.799 ± 0.337 | 4.495 | 4.705 | 5.147 | |
| Day 2 | 4.230 ± 0.377 | 4.017 | 4.240 | 4.350 | |
| Day 5 | 3.850 ± 0.353 | 3.572 | 3.885 | 4.205 | |
| Severe | < 0.001 | ||||
| Day 1 | 4.240 ± 0.188 | 4.120 | 4.220 | 4.330 | |
| Day 2 | 3.908 ± 0.197 | 3.800 | 3.910 | 4.110 | |
| Day 5 | 3.599 ± 0.274 | 3.410 | 3.570 | 3.740 | |
| Zn | |||||
| Moderate | 0.437 | ||||
| Day 1 | 65.285 ± 3.383 | 63.000 | 65.000 | 67.000 | |
| Day 2 | 64.857 ± 3.840 | 62.750 | 65.000 | 67.000 | |
| Day 5 | 66.928 ± 2.644 | 65.000 | 66.000 | 69.000 | |
| Severe | 0.030 | ||||
| Day 1 | 61.200 ± 6.731 | 55.000 | 62.000 | 67.000 | |
| Day 2 | 60.733 ± 6.430 | 54.000 | 62.000 | 66.000 | |
| Day 5 | 61.600 ± 7.058 | 54.000 | 63.000 | 66.000 | |
| NO | |||||
| Moderate | 0.810 | ||||
| Day 1 | 22.691 ± 0.520 | 22.465 | 22.640 | 23.107 | |
| Day 2 | 22.820 ± 0.567 | 22.407 | 22.875 | 23.135 | |
| Day 5 | 22.640 ± 0.513 | 22.370 | 22.630 | 23.100 | |
| Severe | 0.010 | ||||
| Day 1 | 21.148 ± 1.130 | 20.020 | 21.120 | 21.980 | |
| Day 2 | 21.950 ± 0.802 | 21.700 | 22.010 | 22.270 | |
| Day 5 | 22.202 ± 0.472 | 21.740 | 22.190 | 22.480 | |
Evaluation of the Status of Changes in Antioxidant Indices in Patients and Comparison of These Changes in Two Groups of Patients with Severe and Moderate Forms of COVID-19
| Mean ± SD | Percentiles | P-Value | |||
|---|---|---|---|---|---|
| 25th | 50th | 75th | |||
| TNF-α | |||||
| Moderate | < 0.001 | ||||
| Day 1 | 20.667 ± 0.894 | 20.160 | 20.455 | 21.172 | |
| Day 2 | 18.535 ± 0.816 | 17.775 | 18.330 | 19.550 | |
| Day 5 | 17.379 ± 0.889 | 16.600 | 17.075 | 18.310 | |
| Severe | < 0.001 | ||||
| Day 1 | 38.423 ± 6.597 | 32.500 | 41.870 | 43.440 | |
| Day 2 | 29.764 ± 5.662 | 24.500 | 30.810 | 34.500 | |
| Day 5 | 18.666 ± 0.443 | 18.400 | 18.510 | 19.080 | |
| IL-10 | |||||
| Moderate | < 0.001 | ||||
| Day 1 | 13.684 ± 0.394 | 13.3225 | 13.6400 | 14.1125 | |
| Day 2 | 11.890 ± 0.435 | 11.5025 | 12.0000 | 12.2475 | |
| Day 5 | 14.255 ± 0.563 | 13.7850 | 14.2900 | 14.6025 | |
| Severe | < 0.001 | ||||
| Day 1 | 15.713 ± 0.905 | 15.4400 | 15.8300 | 16.3200 | |
| Day 2 | 13.872 ± 0.760 | 13.4700 | 13.8800 | 14.2300 | |
| Day 5 | 16.050 ± 0.670 | 15.7000 | 15.8400 | 16.5200 | |
| Ferritin | |||||
| Moderate | 0.002 | ||||
| Day 1 | 74.214 ± 13.75 | 63.000 | 67.000 | 88.750 | |
| Day 2 | 74.142 ± 12.94 | 64.500 | 67.500 | 88.750 | |
| Day 5 | 72.214 ± 13.43 | 61.750 | 67.000 | 86.750 | |
| Severe | < 0.001 | ||||
| Day 1 | 246.13 ± 47.25 | 248.000 | 258.000 | 264.000 | |
| Day 2 | 126.73 ± 21.04 | 113.000 | 122.000 | 137.000 | |
| Day 5 | 97.266 ± 7.676 | 89.000 | 99.000 | 101.000 | |
Evaluation of Changes in Immunological Indices in Patients and Comparison of These Changes in Two Groups of Patients with a Severe and Moderate Form
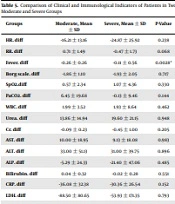
According to Table 5, the changes in fever before treatment and 72 hours after treatment showed a significant difference between the two stages, so the fever decreased more in the moderate group. Changes in SOD before treatment and 72 hours after treatment were significantly different between the two stages, so there was a greater increase in the severe group. GST and FRAP changes before treatment and 72 hours after treatment were significantly different between the two stages, so there is a greater increase in the severe group. Changes in MDA before treatment and 72 hours after treatment indicated a significant difference between the two stages, so there was a decreasing trend in the severe group, whereas there was an increasing trend in the moderate group. Changes in TNF-α before treatment and 72 hours after treatment showed a significant difference between the two stages, so a greater reduction was observed in the severe group. The changes in ferritin before treatment and 72 hours after treatment between the two stages were significantly different, so there was a greater decrease in the severe group. Nitric oxide changes before treatment and 72 hours after treatment were significantly different between the two stages, so there was more increase in the severe group.
| Groups | Moderate, Mean ± SD | Severe, Mean ± SD | P-Value |
|---|---|---|---|
| HR. diff | -16.21 ± 13.16 | -24.87 ± 25.92 | 0.238 |
| RR. diff | 0.71 ± 1.49 | -0.47 ± 1.73 | 0.068 |
| Fever. diff | -0.26 ± 0.26 | -0.11 ± 0.56 | 0.0028* |
| Borg scale. diff | -1.86 ± 1.10 | -1.93 ± 2.05 | 0.717 |
| SpO2.diff | 0.57 ± 2.34 | 1.07 ± 4.36 | 0.330 |
| PaCO2. diff | 6.45 ± 19.68 | -0.13 ± 9.46 | 0.144 |
| WBC. diff | 1.99 ± 3.52 | 1.93 ± 8.64 | 0.462 |
| Urea. diff | 13.86 ± 14.94 | 19.60 ± 21.15 | 0.948 |
| Cr. diff | -0.09 ± 0.23 | -0.45 ± 1.00 | 0.205 |
| AST. diff | 10.00 ± 18.95 | 9.13 ± 18.08 | 0.983 |
| ALT. diff | 33.00 ± 51.13 | 31.00 ± 39.75 | 0.896 |
| ALP. diff | -5.29 ± 24.33 | -21.40 ± 47.06 | 0.485 |
| Bilirubin. diff | 0.04 ± 0.32 | -0.02 ± 0.28 | 0.551 |
| CRP. diff | -36.08 ± 32.38 | -30.36 ± 26.54 | 0.152 |
| LDH. diff | -88.50 ± 80.65 | -53.93 ± 171.35 | 0.793 |
| ESR. diff | -19.21 ± 19.13 | -28.27 ± 24.21 | 0.315 |
| SOD. diff | 0.11 ± 0.36 | 7.58 ± 27.67 | 0.003* |
| GPX. diff | 0.24 ± 0.59 | 0.28 ± 0.34 | 0.647 |
| GST. diff | 0.06 ± 0.04 | 0.33 ± 0.10 | < 0.001 |
| FRAP. diff | 2.13 ± 9.05 | 19.61 ± 9.31 | < 0.001 |
| MDA. diff | 0.44 ± 0.35 | -0.11 ± 0.49 | 0.002* |
| Albumin. diff | -0.95 ± 0.49 | -0.64 ± 0.34 | 0.093 |
| Zn. diff | 1.64 ± 3.61 | 0.40 ± 3.40 | 0.825 |
| TNF-α. diff | -3.29 ± 1.28 | -19.76 ± 6.64 | < 0.001 |
| IL-10. diff | 0.57 ± 0.51 | 0.34 ± 0.72 | 0.238 |
| Ferritin. diff | -2.00 ± 2.96 | -148.87 ± 43.87 | < 0.001 |
| Nitric oxide. diff | -0.05 ± 0.60 | 1.05 ± 1.06 | 0.003* |
Comparison of Clinical and Immunological Indicators of Patients in Two Moderate and Severe Groups
5. Discussion
This study aimed to evaluate the efficacy of methylprednisolone on clinical manifestations, inflammatory biomarkers, and antioxidant changes in patients with COVID-19. We hypothesized that this drug could improve pulmonary function, shortness of breath, immunological markers, and antioxidant markers in these patients. The main findings of the study showed that the immunological and antioxidant parameters of the patients were significantly improved, which confirms our hypothesis.
Based on the results obtained from past studies, infection with SARS-CoV-2 destroys lung tissue cells and stimulation of immune response. This immune response recruits innate immune cells, such as macrophages and monocytes, as the primary response, then T and B cells (adaptive immune cells) are activated against the infection (7). Though in many patients, this primary immune response can effectively stop the infection, in a situation where the patient’s immune system creates a disturbed immune response, a cytokine storm and subsequently pneumonia is created. Investigations have shown that the mortality and severity of COVID-19 are influenced by the increase in the concentration of inflammatory factors such as ferritin, CRP, and cytokine storm created with interleukins, such as TNF-α and Monocyte Chemoattractant Protein-1 (MCP-1). These cytokines can increase the ratio of neutrophils to lymphocytes (8). Infection with SARS-CoV-2 causes the activation of NOD-like receptor protein 3 (NLRP3), as a pattern recognition receptor (PRR), which is responsible for recognition of damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs). The PAMPs are involved in the recruitment and clustering of multi-protein complexes called inflammasomes (9). Inflammation due to NLRP3 activation eventually causes cell death, known as pyroptosis and apoptosis (10). Therefore, the progression of lung damage is often influenced by the activity of type II alveolar cells, endothelial cells, and the activation of the innate immune responses (11). With the activity of alveolar macrophages, the cytokine storm is initiated that stimulates endothelial cells, platelets, and neutrophils, and thus a collection of platelets and neutrophils is created on the surface of endothelial cells (12-18). This isolation of these neutrophilic and platelet structures from pulmonary arteries causes immunothrombosis (19). Convincing evidence suggests that immunothrombosis is a major determinant of the production of microthrombi and microemboli in the capillaries of alveoli circulation, formation of fibrin deposits, and sometimes spread intravascular clot production (20). Therefore, increasing the concentration of neutrophils in the interstitial tissue of the lung and alveoli plays a significant role in creating a cytokine storm and tissue damage, leading to the deterioration of the patient’s clinical conditions and ARDS (21).
Therefore, tissue damage caused by SARS-CoV-2 infection (especially lung tissue) is affected by several mechanisms and factors. However, studies have shown that Reactive oxygen species (ROS) is one of the factors that play a pivotal role in the initiation and progression of these inflammatory mechanisms (22). Another effective factor in causing inflammation is NLRP3 (23). However, other pathological pathways may be involved in the induction of NLRP3. The development of this inflammation caused by NLRP3 is influenced by the expression of IL-18 and IL-1β due to the stimulation of NF-κB (24). Indeed, when the innate response fails to control infection, NLRP3 overactivity leads to mitochondrial dysfunction, DAMPs release, and increased pyroptosis, leading to virus spread and extensive destruction of damaged tissues (25).
Today, many antiviral treatments are based on the effect on intracellular redox pathways. Studies have shown that respiratory viral infections, especially SARS-CoV-2, inhibit nuclear factor erythroid 2-related factor 2 (NRF2) and activate nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways despite disrupting ROS production, leading to inflammation and oxidative damage. Therefore, examining NRF2 activators in patients with COVID-19 may be of importance. The clinical benefits of dexamethasone, hydrocortisone, or methylprednisolone have been evaluated in previous studies. The review of these studies showed that the period of treatment with corticosteroids ranged from three to 14 days, and the dosage of the drug was gradually increased. The effectiveness of dexamethasone compared to methylprednisolone showed different results (26). Also, the clinical conditions of the patients in different studies showed various changes and might have been affected by factors such as the severity of the disease, the type of corticosteroid, the dosage, and the statistical power of the study (27).
The results of studies on the effectiveness of methylprednisolone have not directly indicated the positive effects of this drug. A study evaluated methylprednisolone by examining 393 patients with COVID-19 at a dose of 1 mg/kg compared with placebo. The result of this study confirmed the positive role of methylprednisolone on 28-day mortality of patients. However, it did not affect virus clearance (28). In another study conducted on 85 patients with moderate to severe COVID-19, the patients received 40 mg of methylprednisolone for three days, and 20 mg was given to the patients three days later. The researchers found that methylprednisolone could reduce the mortality and severity of the disease in these patients (29). Also, a quasi-experimental study of patients with moderate to severe COVID-19 who used 0.5 - 1 mg of methylprednisolone for three days confirmed the effect of this drug on reducing mortality compared to the control group in which the patients were transferred to the ICU (30). The timing of the use of corticosteroids as a key factor in the treatment of the onset of shortness of breath was also supported. These measures appeared to prevent the progression of the disease associated with the host pro-inflammatory responses. On the other hand, a retrospective cohort study that examined 205 patients with severe phases of COVID-19 showed that taking 80 mg of methylprednisolone daily did not significantly change the mortality of patients (31).
Although observational and randomized trial data generally support the role of corticosteroids in the treatment of severe COVID-19, most studies have linked this advantage to the need for respiratory support (32). In addition, examining the progress of the disease in different people has shown that different clinical responses and clinical conditions of each patient can affect the type of reaction to corticosteroids. Thus, the benefits of corticosteroid therapy may also depend on the degree of inflammation (33). The present study has limitations, such as being monocentric with observational and retrospective nature. However, this study was unique to the patient population in Iran because it focused on a subset of patients developing severe inflammatory syndrome. According to the present information, no study has compared the effect of methylprednisolone treatment based on the degree of severity on immunological and antioxidant indicators. The results obtained from our study show that the effectiveness of methylprednisolone can be beyond what has been discussed in other studies.
5.1. Conclusions
The use of methylprednisolone by improving the balance of antioxidants and immunological factors in patients with COVID-19 improves some clinical indicators in these patients. Thus, methylprednisolone can be considered a drug of choice in patients with moderate to severe COVID-19.