1. Background
China informed the World Health Organization of a pneumonia outbreak with an unknown cause on December 31, 2019 (1). It was eventually determined that severe acute respiratory syndrome coronavirus 2 (SARSCOV2) was the source of the worldwide pandemic on March 11, 2020 (2), and Iran reported its first confirmed cases in February 2020 (3). The virus rapidly spread in Iran, causing tremendous damage to adults and little to no formal studies of the pediatric and newborn populations. As there were no diagnostic tools to recognize SARSCOV2 during the initial months of the crisis, no diagnostic kits were assigned to the neonatal population. Hence, the original reports of influenced neonates were from maternity hospitals (4). This pattern was observed in other countries, leading the WHO, CDC, and AAP to issue protocols and guidelines about control and prevention during the prenatal period, labor, and after delivery (2). Despite the growing understanding of SARSCOV2 and the strict following of all regulations and rules, the virus' variants and mutations still infect children, particularly newborns. Most neonatal SARSCOV2 infections are caused by postnatal exposure, making up 70% of cases, while vertical transmission, either congenital or intrapartum, adds up to 30% (5, 6). Postnatal exposure can occur through breastfeeding, maternal respiratory droplets, or contact with another infected family (7). In our experience, the clinical attributes of postnatal infection in neonates may be distinctive from those who contract the illness through perinatal transmission. As soon as we started using RT-PCR in our NICU to test neonates, we recorded any neonates that tested positive for SARSCOV2.
2. Objectives
The current investigation sought to recognize, explain, and document certain potential characteristics of neonates who were infected after birth by horizontal virus transmission in a referral hospital in Iran.
3. Methods
3.1. Study Design and Patients
This observational cohort study was conducted covering the period of October 2020 to March 2022 in the NICU and neonatal ward of the Children's Medical Center in Tehran, Iran, which spanned from the beginning of the Wuhan variant from China to the emergence of the Omicron variant from South Africa. We tested RT-PCR on infants admitted to the NICU or Neonatal ward with respiratory symptoms, fever, hypothermia, cyanosis, poor feeding, milk intolerance, and vomiting based on the clinical manifestation of infected neonates from the Saeedi et al. study (8). We additionally sent RT-PCR tests if the neonate was admitted for an illness not caused by an infectious agent, but the mother was symptomatic of SARSCOV2 infection.
3.2. Inclusion Criteria
For this study, all term neonates under 28 days old and preterm neonates with a corrected gestational age under 42 weeks or body weight below 3500 grams were included if they became infected postnatally with SARSCOV2 due to horizontal virus transmission from their mother, father, or another person with a positive PCR test for the virus.
Neonates with a maternal infection near the time of birth were included to study if the neonates had a negative test at age 24 - 48 hours and a positive test at ≥ 48 hours as early postnatal infection based on the definition and categorization of the timing of mother-to-child transmission of SARSCoV2; modified WHO guidelines (7).
3.3. Exclusion Criteria
All neonates with negative RT-PCR results, neonates with congenital or intrapartum SARSCOV2 infections based on definition and categorization of the timing of mother-to-child transmission of SARSCoV2; modified WHO guidelines (7, 9), and neonates with positive blood culture were excluded from the study even if they had positive RT-PCR tests, positive index cases, or the above-mentioned.
3.4. Lab Tests
Quantitative RT-PCR samples were taken from the neonates’ nasopharyngeal swabs, and in suspicious cases in which neonates had symptoms but a negative result, we repeated the test after 24 hours (10). We used the Pishtaz Teb Coronavirus RT-PCR kit (98001). The Coulter counter method was used for CBC (complete blood count), and C-reactive protein (CRP) was checked by a quantitative test.
3.5. Data Collection
After making the issue's significance clear and getting consent, the hospital manager gave the go-ahead to carry out research using patients' lab results. All the data concerning admitted neonates who met the criteria were gathered through clinical records, the hospital HIS system, and discussion with parents or their caregivers. At the end of each month, postnatal infections were selected, and the frequencies of patients with SARSCOV2 infection were matched with the number of patients reported by the hospital admission unit to investigate any missing cases. This includes information such as population statistics when the illness started, physical symptoms, how intense the respiratory problem is, relevant lab tests, the original case, the type of treatment received, and the results.
3.6. Statistics
We analyzed our data with SPSS (IBM Corp. Released in 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp). Descriptive statistics, including frequencies, percentages, mean, standard deviation, median, and Interquartile range (IQR), were employed to analyze the data.
3.7. Ethics
This study was evaluated by the Research Ethics Committee of Children's Medical Center-Tehran University of Medical Sciences. The study abided by ethical principles and the national norms and standards for conducting medical research in Iran. Approval ID: IR.TUMS.CHMC.REC.1400.187.
4. Results
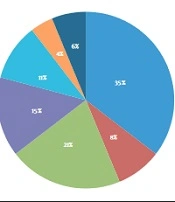
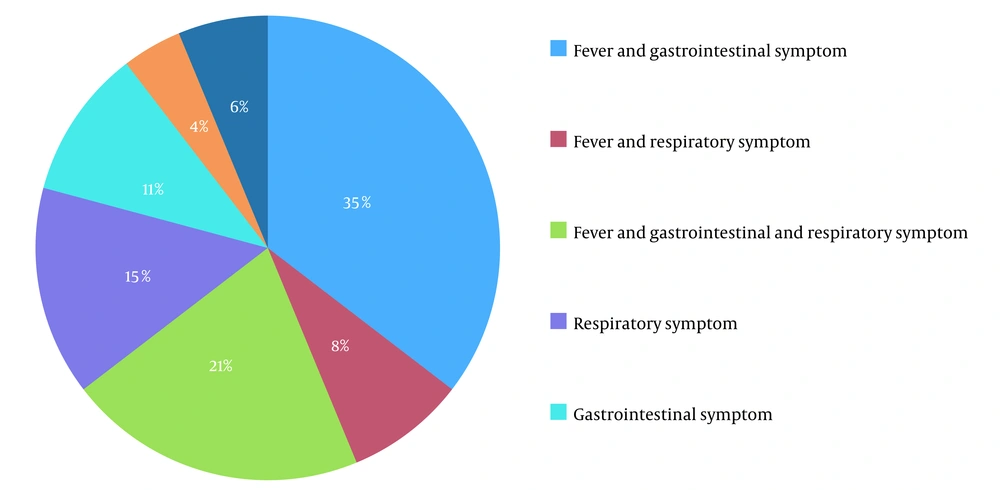
We identified a total of 55 neonates as having postnatal SARSCoV2 infections. Twelve neonates had a gestational age of fewer than 37 weeks (22%) (Table 1). The most common sign of admission was a fever, which was present in 35 cases (61%). Most of the time, they came with other symptoms (Figure 1). Diarrhea and vomiting (32%) were the most typical gastrointestinal symptoms and the second symptom. The third most frequent symptom in neonates was poor feeding (30%). Coughing was the most common sign of respiratory illness (27%), and respiratory distress was seen in 13% of neonates, determined by having a Respiratory Distress Score (RDS) of 5 or more. The occurrence of NEC in neonates was 18%, and preterm babies made up 30% of those. All cases of NEC were stage 1, which improved with medical treatment. In 38% of newborns, the lymphocyte count was less than 3000, which was defined as lymphopenia (11). There were 19 cases of neutropenia, representing 34%. Five neutropenic patients had severe levels (< 500 µ/L). Platelet count in all cases was normal. The average concentration of CRP was 8 ± 20 mg/L, with a range of 0.4 to 120 mg/L, twelve patients had CRP levels of more than 6 mg/L, and 58% of them had CRP of more than 30 mg/L (Table 2).
| Variables | No. (%) | Minimum | Maximum | Mean ± SD |
|---|---|---|---|---|
| Sex (male) | 26 (47.3) | |||
| Gestational age, w | 31 | 42 | 37.65 ± 1.792 | |
| Preterm | 12 (22) | |||
| Term | 43 (78) | |||
| C/S | 38 (69) | |||
| Birth weight, gr | 1510 | 4300 | 3034.23 ± 551.822 | |
| 1000 - 2000 | 2 (3.6) | |||
| 2000 - 3000 | 24 (43.6) | |||
| > 3000 | 29 (52.7) | |||
| Transmission of SARSCOV2 to neonates (index cases) | ||||
| Mother | 35 (63) | |||
| Father | 7 (12) | |||
| Other members of the family | 4 (7) | |||
| Unknown | 10 (18) |
| Lab Tests | Mean ± SD | Median (IQR) | Range |
|---|---|---|---|
| C-reactive protein (mg/L) | 8 ± 20 | 2 (1 - 3) | (0.4 to 120) |
| PLT (µL) | 325.712 | 315.000 | (116 to 725) |
| White blood cell (µL) | 7515 ± 3213 | 7020 (5240 - 9300) | (1374 to 19700) |
| Neutrophil count (µL) | 1766 ± 1034 | 1867 (1026 - 2359) | (94 to 5226) |
| Lymphocyte count (µL) | 4149 ± 2067 | 3823 (2645 - 5361) | (398 to 9456) |
Two newborns had a co-infection of their urinary tract. No one of the neonates had a positive blood culture result following the covid infection. Within the studied population, 82% (46 neonates) had a positive index case at the time of entry, and these were either in direct or indirect contact with parents or relatives who tested positive recently. Of thirty-five neonates, 63% were in close contact with a PCR-positive mother, while 7% and 12% were in contact with a PCR-positive father and another PCR-positive caregiver, respectively. We did not administer antivirals, anticoagulants, or corticosteroids as treatment; however, due to the adverse laboratory results and the overall condition of patients, we had to start empirical antibiotics in the majority of cases; antibiotic therapy in most cases had a short duration. Moreover, 43 infants (78.2%) did not need supplementary oxygen. Nonetheless, three newborns had to be mechanically ventilated during their treatment, four needed non-invasive ventilation, and five babies were given extra oxygen. All neonates were discharged without complications, and there were no fatalities among the studied population.
5. Discussion
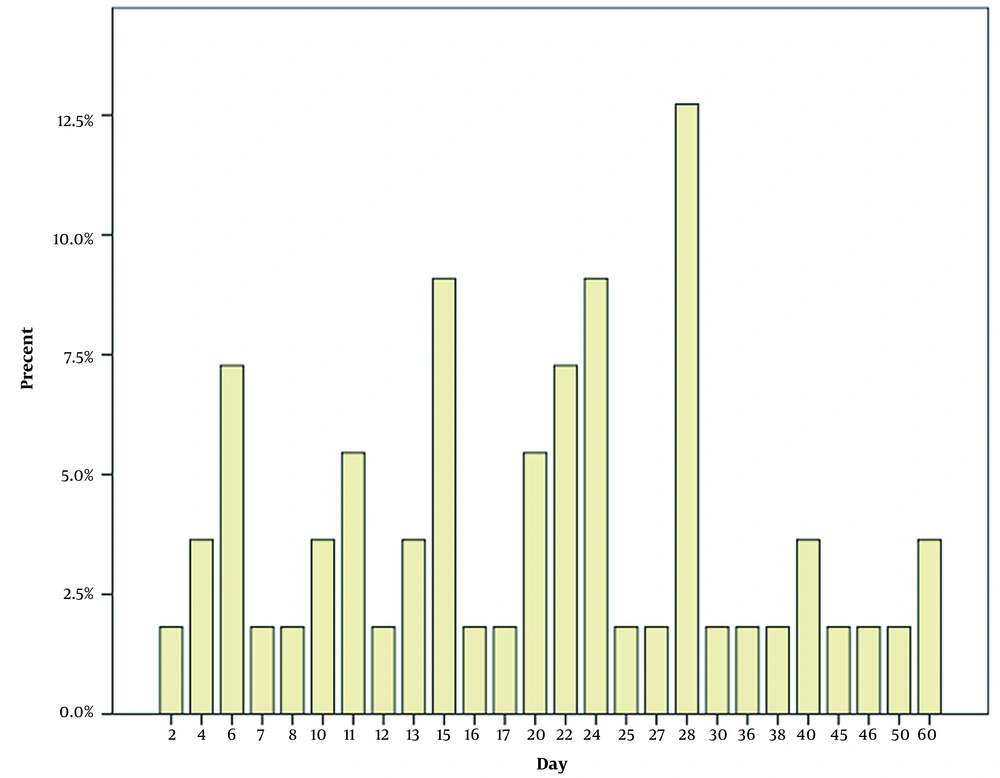
Our research examines postnatal SARSCOV2 infections in neonates admitted to our hospital. Fever was the most frequent clinical presentation, with other symptoms typically accompanying it. The most frequent symptoms following fever were those related to the gastrointestinal system (Figure 1). Mithal and the other researchers discovered that fever was a more commonly observed symptom than what was seen in our study (77.8%). In the 18 patients studied, one bacterial urinary tract co-infection case was discovered, which was similar to the two cases in our study (12). As a result, it may be worth researching the correlation between urinary tract infections and SARSCOV2 infections. Gale et al. conducted a study in the UK and discovered that fever, poor feeding, and vomiting were more recurrent in neonates than what was found in our research. Additionally, the UK study had a greater prevalence of respiratory distress (13). This study also found that 42% of cases had severe disease according to Dong et al.’s criteria (14). Thirty-three percent of newborns needed some kind of respiratory help, and 20% needed both invasive and non-invasive ventilation, which was more often required than in our study. In addition, we included neonates admitted to the hospital; we did not include asymptomatic or mildly symptomatic outpatient infants in our study. These differences might have been related to older neonates in postnatal infection. Our patients were affected only by postnatal exposure, unlike Gale et al.'s (13) study of congenital, intrapartum, and postpartum cases. Perhaps the association of perinatal SARSCOV2 infection with primary diseases such as RDS causes respiratory symptoms to worsen. Therefore it needs more studies to compare the severity of the disease in postnatal with perinatal infection. At the start of the SARSCOV2 pandemic, although antiviral medications were administered to adults and lower dosages for children, including preterm infants (15), none of the participants in our study were provided antiviral, anticoagulant or corticosteroid treatments, even in the most serious cases. Supportive treatment was successful in treating all of our patients, so the role of these medications in the treatment of SARSCOV2 infection in newborns is something that requires further exploration (16). Our research revealed that 82% of the neonates had contact with someone from an infected household, emphasizing the need for social distancing during virus outbreaks. Although the global spread of the virus has demonstrated that, in addition to direct contact with respiratory droplets, contact with people who are not exhibiting any symptoms is another primary cause of the transmission of illness. Therefore, the presence of caregivers in health centers and hospitals during the newborn stage increases the chances of transmitting SARSCOV2. Furthermore, this could explain the larger number of infections reported in the early days of life (Figure 2). The demographic data demonstrates that term neonates are more likely to be affected than preterm neonates (Table 1). Villar et al. noticed that the gestational age at delivery was shorter in women with SARSCOV2 infection as compared to women who did not have the disease (17). Sorsa A. and other studies have shown that sepsis in preterm neonates is more prevalent than in term neonates (18). Therefore we suggest designing a study to compare the prevalence of bacterial infection with that of SARSCOV2 infection in preterm neonates. Out of the patients, 20% had CRP levels above 6 mg/L, and 7 of them had levels higher than 30 mg/L, without any bacterial infection found in complete sepsis workup, except in one case with CRP of 120 mg/L that showed bacterial urinary tract co-infections. In the study conducted by Ali, the average concentration of CRP was seen to be higher in more serious conditions, and an increase of one unit in CRP could result in a 5% rise in serious events in adult SARSCOV2 patients (19); however, this relationship was not noted in newborns in our study. Additional research needs to be conducted to validate it. This research discovered that some of these patients experienced transient neutropenia during hospitalization. Few reports of neutropenia have been found in neonatal studies with infection (20). Bone marrow suppression or peripheral destruction can be caused by some viral infections, which are common causes of neutropenia (21). It's possible that postnatal SARSCOV2 infection can lead to neutropenia, just like many other viral infections. According to the White et al. report, neutropenia was observed in a Colorado Neonatal Intensive Care Unit when community-acquired SARS-CoV-2 was present (22). Liu et al. discussed how the intense release of inflammatory cytokines could heighten inflammatory factors and the neutrophil population, specifically in serious infected adult cases (23). On the other hand, neutropenia appears more often in infants under six months old than in infected older children (24).
5.1. Conclusions
Our research showed that fever was the most prevalent symptom when admitted. The high rate of neutropenia and/or a high CRP value among these patients and their general malaise led to the premature start of antibiotic treatment. Further observational studies are necessary to make an accurate diagnosis and treatment.