1. Background
Thalassemia syndrome is a variant of hemoglobinopathies. According to the World Health Organization, it is globally one of the most common causes of anemia, with a prevalence of 7% in the world (1-4). The dysfunction in beta-chain hemoglobin synthesis leads to chronic anemia, requiring frequent, lifelong blood transfusion for survival (4, 5). Thalassemia falls into transfusion-dependent thalassemia (TDT) or thalassemia major (TM) and non-transfusion-dependent thalassemia (NTDT) or intermediate thalassemia (TI) (1). A consequence of regular blood transfusion is increased ferritin and iron deposition in vital organs such as the heart, liver, and endocrine glands (4, 6, 7).
Coronavirus disease 2019 (COVID-19) is an infectious disease of the respiratory system that has been a pandemic since February 2019. The disease's prevalence, severity, and prognosis appear to vary in children and adults or normal people with underlying diseases (8).
Coronavirus is a group of related viruses that cause disease in mammals. Coronavirus causes respiratory infections that range from mild (e.g., the common cold) to severe respiratory symptoms in humans. The most common symptoms in humans are fever, cough, difficulty breathing, and other reported symptoms such as fatigue, anorexia, headache, body aches, sore throat, chills, nausea, vomiting, diarrhea, and anosmia. Hepatic and neurological symptoms also might appear in some cases (9-12).
Like the general population, thalassemia patients are at risk of being infected with viruses and developing acute diseases. On the one hand, blood transfusion and immunomodulation caused by blood transfusion and splenectomy in approximately 50% to 60% of thalassemia patients put them at a higher risk of COVID-19. However, the available information about COVID-19 in patients with beta-thalassemia seems limited.
Recent studies at the University of Sichuan also showed that ORF8 and glycoproteins can bind to porphyrins. At the same time, the orf1ab, ORF10, and ORF3a proteins can attack each other in combination in beta-1. Therefore, iron is separated from hemoglobin, and porphyrin is formed. This attack leads to decreased hemoglobin and hypoxia (13, 14).
Rahimizadeh et al. studied 9 hospitalized patients infected by COVID-19. Common symptoms included fever, chills, cough, and tachypnea. Three patients had leukopenia and lymphopenia. A COVID-19 RNA test was positive in these three patients. All nine patients were diagnosed with increased CRP protein and ESR and received supportive care and antibiotics for 6 days. Also, COVID-19 pneumonia was treated in these pediatric patients without a ventilator or coronary artery therapy (9).
Another small cohort study in Italy showed fewer thalassemia patients infected with COVID-19 (11 TM and TI patients) than the general population. They experienced mild to moderate disease, although all patients had some form of thalassemia. In addition, mortality was not high (15).
This study included beta-thalassemia patients from March 2019 to the end of December 2020, as introduced by thalassemia associations. The patients had suspected symptoms, were hospitalized and non-hospitalized, or underwent additional tests.
2. Objectives
Our study aimed to evaluate epidemiological data of beta-thalassemia patients with different health conditions for mortality and severity of their COVID-19 infection. Thus, the prevalence and the risk of COVID-19 in thalassemia patients according to their severity were assessed in different provinces and their families.
3. Methods
This is a cross-sectional study with an ethical code of IR.TMI.REC.1399.013. The data were collected in collaboration with the thalassemia associations. We collected provincial information using electronic checklists. The statistics of beta-thalassemia and COVID-19 patients (positive test) were obtained from the database. Screening information was received from the Ministry of Health database using these patients and their families' national registration codes. A questionnaire was designed to collect demographic, clinical, and paraclinical data and treatment responses using their history, medical charts, and laboratory tests. If the patients had flu-like or atypical symptoms, the COVID-19 PCR test was performed. Finally, the statistics of patients in each province were compared with the prevalence of the disease in the general population. Meanwhile, the total national statistics of COVID-19 in thalassemia patients were compared among their family members, and differences were used to interpret the results.
The study was conducted on the population of beta-thalassemia and those infected with COVID-19 across the country from March 2019 to the end of December 2020. A random sampling method was used, and information was extracted from checklists and electronic databases. If the patients' family information was also available, it was also gathered. We considered some factors for the severity of the patient's infection, such as ferritin, iron overload in the heart and liver, cardiac dysfunction, pulmonary hypertension, diabetes, and splenectomy, to evaluate the mortality and morbidity in this study population. The statistical analysis was done using SPSS (version 21), and a P-value < 0.05 showed a significant correlation. All data were analyzed by Pearson chi-square statistical test.
4. Results
The number of thalassemia patients in this study was 19387. Of them, 442 patients were infected with COVID-19, according to the information obtained from the relevant associations. Also, 419 patients had positive PCR tests, while 23 did not test positive, but there was a positive PCR test result in their families. Most of them had suspected clinical symptoms. Based on the patient's sex, 50.9% were female, and 49.1% were male (225 females vs. 217 males). The age range of the studied patients was 16 - 67 years old (36.98 ± 8.519). Their mean weight was 62.33 ± 13.66 kg, and their height was 163.11 ± 12.287 cm. In addition, 81% of the patients were TM, 17.4% were TI, 1.1% had Alpha thalassemia, and 0.5% were sickle cell.
Based on the information obtained from the patients' clinical files, pharyngeal samples showed that 419 out of 442 patients (94.8%) had positive PCR tests for COVID-19, while for 23 patients (5.2%), the COVID-19 PCR test was negative, but they showed suspected COVID-19 symptoms. According to the statistics collected by thalassemia associations in each city, the provincial prevalence of the patients across the country is presented in Table 1.
| Province | Provincial Statistics, No. (%) | COVID Infected Thalassemia (%) | Total Number of Thalassemia Patients |
|---|---|---|---|
| Alborz | 15 (3.3) | 4.6 | 320 |
| Ardabil | 1 (0.2) | 1.2 | 80 |
| Azerbaijan Gharbi | 5 (1.1) | 7.6 | 65 |
| Azerbaijan Sharghi | 11 (2.4) | 9.1 | 120 |
| Bushehr | 3 (0.7) | 0.9 | 315 |
| Charmahalobakhtiari | 9 (2) | 2.8 | 311 |
| Darab | 2 (0.5) | 1 | 200 |
| Esfahan | 3 (0.7) | 0.3 | 935 |
| Ghazvin | 4 (0.9) | 3.1 | 127 |
| Gheshm | 1 (0.2) | 0.8 | 117 |
| Ghom | 18 (4.07) | 13.8 | 130 |
| Guilan | 21 (4.7) | 1.5 | 1370 |
| Golestan | 1 (0.2) | 0.1 | 600 |
| Hamedan | 12 (2.7) | 15 | 80 |
| Hormozgan | 27 (6.1) | 1.9 | 1400 |
| Ilam | 9 (2) | 7.5 | 119 |
| Kerman | 31 (7) | 3.1 | 1000 |
| Kermanshah | 12 (2.7) | 6 | 200 |
| Khorasan | 6 (1.4) | 1.5 | 378 |
| Khuzestan | 36 (8.1) | 1.6 | 2198 |
| Kohkiloye | 3 (0.6) | 1.01 | 295 |
| Lorestan | 16 (3.6) | 11.2 | 142 |
| Mazandaran | 16 (3.6) | 3.6 | 2540 |
| Sanandaj | 3 (0.7) | 0.9 | 150 |
| Semnan | 1 (0.2) | 2 | 85 |
| Shiraz | 1 (0.2) | 0.1 | 800 |
| Sistan and Baluchestan | 19 (4.2) | 0.5 | 3210 |
| Tehran | 147 (32.2) | 8.1 | 1800 |
| Yazd | 11 (2.5) | 3.6 | 300 |
| Total | 442 (100) | 2.27 | 19387 |
The patient's history was checked for fever symptoms. The results showed that 98% of the patients complained of fever. In addition, 54% (242 out of 442) had obvious cough symptoms, and 50.45% (223 out of 422) had both fever and cough symptoms. The prevalence of symptoms in thalassemia COVID-19 infected patients is presented in Table 2.
| Symptoms | Frequency (%) |
|---|---|
| 10% pulmonary involvement | 11 (2.5) |
| 3% pulmonary involvement | 11 (2.5) |
| 50% pulmonary involvement | 1 (0.2) |
| 60% pulmonary involvement | 1 (0.2) |
| Bone pain | 36 (8.1) |
| Bone pain and fever | 66 (14.9) |
| Bone pain, fever, and cough | 63 (14.4) |
| Bone pain, cough, fever, and diarrhea | 12 (2.8) |
| Dizziness, headache, and cough | 11 (2.5) |
| Dyspnea | 33 (7.5) |
| Headache | 9 (2.0) |
| Headache, fever, and diarrhea | 76 (17.2) |
| Loss of smell, fever, and bone pain | 66 (14.9) |
| Loss of taste and smell, fever, and headache | 9 (2) |
| Sore throat, fever, cough, and diarrhea | 31 (7.1) |
| Thrombosis | 1 (0.2) |
| Twice corona | 3 (0.7) |
| Total | 442 (100.0) |
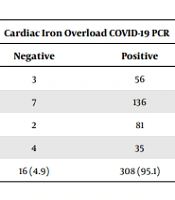
We found that 205 (46.4%) patients were splenectomized. Also, 192 (93.65%) out of the 205 splenectomized patients and 227 (95.78%) out of 237 non-splenectomized patients had positive PCR tests for COVID-19. The mean FBS of the studied patients was 103.61 ± 47.213 (65 - 349), and the mean of ferritin was 2627.65 ± 4603. Also, 599 (43 - 7000). The mean ejection fraction (EF) was evaluated in echocardiography, showing 54.85 ± 7.226 (20 - 72). Pulmonary artery pressure (PAP) was 29.61 ± 11.268 (28 - 110). Tables 3 and 4 show the diabetes prevalence and liver and heart iron overload in infected patients.
| COVID-19 PCR | Diabetes Mellitus | No. (%) | |
|---|---|---|---|
| No | Yes | ||
| Negative | 0 | 1 | 1 (2.1) |
| Positive | 33 | 14 | 47 (97.9) |
| Total, No. (%) | 33 (68.8) | 15 (31.3) | 48 (100.0) |
| Cardiac Iron Overload COVID-19 PCR | Total, No. (%) | Liver Iron Overload COVID-19 PCR | Total, No. (%) | |||
|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | |||
| Normal | 12 | 209 | 221 (70.2) | 3 | 56 | 59 (18.2) |
| Mild | 3 | 84 | 87 (27.6) | 7 | 136 | 143 (44.1) |
| Moderate | 0 | 6 | 6 (1.9) | 2 | 81 | 83 (25.6) |
| Severe | 0 | 1 | 1 (0.3) | 4 | 35 | 39 (12.0) |
| Total, No. (%) | 15 (4.8) | 300 (95.2) | 315 (100.0) | 16 (4.9) | 308 (95.1) | 324 (100.0) |
We also checked the results of the family members. Table 5 summarizes this information. It seems that 25.6% of the family members suffered from COVID-19 complications.
| Number of Infected Family Members | Family Members (Alive) | No. (%) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 0 | 18 | 10 | 22 | 12 | 0 | 62 (14) |
| 1 | 0 | 43 | 33 | 16 | 0 | 92 (20.8) |
| 2 | 0 | 5 | 13 | 39 | 5 | 62 (14) |
| 3 | 0 | 0 | 1 | 18 | 19 | 38 (8.6) |
| 5 | 0 | 0 | 0 | 0 | 14 | 14 (3.2) |
| All | 6 | 31 | 44 | 20 | 12 | 113 (25.6) |
| Total | 24 (5.4) | 89 (20.1) | 113 (25.6) | 105 (23.8) | 50 (11.3) | 442 (100) |
Out of the 442 patients, 14 (3.2%) died. Four of the dead patients had a ferritin level of more than 5,000. The ferritin level in the rest of the patients was about 2,000 and 3,000. Three of them had a history of diabetes. Ten patients presented with medical complaints of cough and shortness of breath, and in the case of increased PAP, one had a PAP of about 110, the other one had a value of 55, and the third one was 40 (normal range 28 to 30).
There was a significant correlation between COVID-19 mortality and increased PAP (P value = 0.003) in our thalassemia patients. There was also a significant correlation between COVID-19 mortality and ferritin level (P value = 0.005). In contrast, there was no significant correlation between COVID-19 mortality and respiratory symptoms like cough (P value = 0.30) nor between COVID-19 mortality and PCR test results (P value = 0.74). We also found no significant correlation between COVID-19 mortality and diabetes (P value = 0.47). In our study, 2.5% of the PCR test results were negative, while the patients had similar COVID-19 symptoms. This means a low false negative percentage.
5. Discussion
Following the COVID-19 crisis in the world, the first cases in Iran were officially confirmed in February 2019 by the Ministry of Health. The Ministry of Health announced that the disease's first peak occurred from late March to April 2019 (16). By April 2019, provincial information was not yet provided. Therefore, we began to collect data from the Iranian Thalassemia Association and other provincial associations at different times. We compared the statistics of the general population, registered by the national registry system of the Ministry of Health, to the statistics of our studied patient population.
In February 2019, the total number of people infected by COVID-19 was approximately 1,550 142 (based on definite PCR tests). Considering the population of Iran, which is 84 million, it comprises about 1.8% of the general population (prevalence = 0.0184; 95% CI: 0.0183 - 0.0185). A total of 59 264 deaths from COVID-19 were reported, accounting for about 3.8% of deaths in infected patients (mortality rate = 0.038; 95% CI: 0.037 - 0.038). By the end of our study, about 442 patients were infected with COVID-19. This incidence accounted for about 2.16% of whole patients, which was slightly higher than corresponding values in the general population (at that time, prevalence = 0.02; 95% CI: 0.019-0.020). Prior to general vaccination, most deaths occurred in the elderly population with underlying diseases such as diabetes, heart disease, and respiratory diseases like asthma. According to a study by the Tehran University of Medical Sciences, most patients who died were 60 years old (17).
Thalassemia syndromes are the most prevalent hemoglobinopathy disorder in the world, and Iran is located on the thalassemia belt. Therefore, these syndromes are critical in our country. Patients who suffer from thalassemia seem to be at higher risk for developing severe complications of COVID-19. Hence, the outbreak of COVID-19 has an important influence on our patients, their families, and physicians. The prevalence of this disease means that thalassemia patients are more prone to COVID-19, while the mortality rate seems insignificant. Since such a study was conducted on patients admitted to hospitals, and our study included both outpatients and inpatients, the results cannot be compared very conclusively. There was no gender difference in our patients, as presented in the results. The mean age of our patients was less than that of the general population affected by COVID-19 since the community of thalassemia patients is still young (18-21).
Morbidity was 3.2% in our studied thalassemia patients; four had a ferritin level of more than 5000 ng/dL. There was a significant relationship between COVID-19 mortality rate and increased PAP and ferritin (P value = 0.003 and P value = 0.005, respectively), which seems to be a severity condition in these patients infected with COVID-19.
A review study by Hashemieh and Shirvani presented the management strategies and some important recommendations for Iranian patients with thalassemia. They concluded that there are many unanswered questions in managing these patients due to the small number of thalassemia patients infected with COVID-19 (21).
Rahimi et al. in Iran also published a nationwide review with the key terms of β-thalassemia/thalassemia and COVID-19. They showed that in thalassemia patients, comorbidities might make them vulnerable to viral infection. Furthermore, cardiac or hepatic hemosiderosis in these patients affects the hypothalamus or adrenal glands with consequent adrenal hypofunction, which might limit the ability to fight infections (22).
In a study by Kumari et al. on the clinical pattern of severe acute respiratory syndrome of COVID-19 in thalassemia patients, they found that symptomatic individuals were significantly fewer among the cases (n = 12) than controls (n = 22) (P = 0.009), which seems to be different from the pattern in Iranian thalassemia patients. This might be due to the differences of our societies in prevention more than physiological defects of the thalassemia patients (23).
5.1. Conclusions
According to the statistics of our thalassemia patient population, the prevalence of COVID-19 death seems to be significantly different. Due to chronic anemia of the thalassemia patients, they need regular blood transfusions and iron chelation. Iron deposition side effects like diabetes or heart failure, which are considered a threat to immune modulation, might lead to the severity of COVID-19 and increase the probability of death in these patients in case of no vaccination. Moreover, patients with no spleen are at a higher risk of COVID-19 infection. However, following preventive health tips and vaccinating all patients with reliable vaccines are the best ways to prevent COVID-19 in thalassemia patients.