1. Introduction
Crimean Congo Hemorrhagic Fever (CCHF) is a tick-borne disease caused by a Nairovirus of the Bunyaviridae family (1, 2). The CCHF virus was first described in the Crimean Peninsula (3), and in 1967, it was isolated from a sick child in the Democratic Republic of Congo; hence the name, CCHF virus (4). The virus is widely distributed throughout the world, especially in Africa, the Balkans, the Middle East, and Asia, and is most frequently reported from Turkey, Russia, Iran, Pakistan and Afghanistan (5-7). The hosts of the CCHF virus include a wide range of wild and domestic animals such as cattle, sheep, and goats. In Iran, the virus was first identified in the early 1970s, and the CCHF antibody was detected in serum samples of livestock and other animals (8). Then, many researchers started studying the serology and epidemiology of the disease in Iran. The genus Hyalomma is the primary vector of CCHF (2). When CCHF-infected ticks bite animals, the virus remains in their bloodstreams for almost a week, and then the tick-animal-tick cycle continues when another tick bites. Principally, transmission to humans occurs via the bite of infected ticks or animal blood fluid and tissues, making livestock handlers the usual victims (2).
The possible causes of CCHF include alterations in agricultural and animal husbandry practices involving domestic animals and climate changes (9). In some areas, livestock trade and the movement of animals from place to place, nomadic lifestyles and human movement in search of water and fresh pastures, and inadequate health care systems may influence the dynamics of host-tick-virus leading to the transfer of CCHF virus-infected ticks to non-endemic areas (10). The incubation period of CCHF depends on the mode of transmission of the CCHF virus and is usually within 1 to 3 days, with a maximum of 9 days (2). This period after contact with infected blood or tissues is usually 5 to 6 days, with a maximum of 13 days.
The common symptoms are fever, myalgia or muscle ache, dizziness, neck pain and stiffness, backache, headache, sore eyes, and photophobia (sensitivity to light). Also, there are nausea, vomiting, diarrhea, abdominal pain, and sore throat in the early stages, followed by sharp mood swings and confusion (2). After 2 to 4 days, an agitated CCHF patient may experience depression, lassitude, sleepiness, and abdominal pains, which may affect the upper right quadrant of the human body, with noticeable liver enlargement (4). Patients with Hepatitis and those who are very ill may experience rapid kidney deterioration, sudden liver failure, or pulmonary failure after the fifth day of illness (2).
At a time when the COVID-19 pandemic still attracts a great deal of attention, CCHF, as an endemic infectious disease, poses a serious threat to many healthcare systems. This is because both diseases in the early stages have similar symptoms but with different modes of transmission, incubation periods, and mortality rates (11). As a result, diagnosis at some stages can be very difficult, and occasionally, co-infection, leading to ignoring this issue, may occur (11-13). This study reports the first confirmed but misdiagnosed CCHF case in the setting of an ongoing COVID-19 pandemic from Ravansar County of Kermanshah province in Iran.
2. Case Presentation
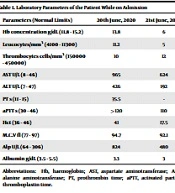
On 18th June 2020, a previously healthy female of 32 years of age from the Kermanshah province was reported to have experienced nausea, vomiting, fever, headache, and myalgia. These symptoms were accompanied by reduced platelet counts, decreased consciousness, and an international normalized ratio (INR) >6 (Table 1). She was a housekeeper who kept sheep and never traveled abroad. On initial examination, the patient showed no signs of petechiae, purpura, or ecchymosis. Then, she was referred to Imam Reza Hospital in Kermanshah on 20th June 2020 but was initially diagnosed with COVID-19, leading to her hospitalization. On the same day, the patient showed symptoms of CCHF. The symptoms were detected based on the epidemiological findings in the guidelines of the Iranian National Institute of Virology. Tick bite crashed tick or direct contact with infected human or animal blood or tissue, or a trip to an infected rural area, clinical signs (fever > 38°C, headache, myalgia, vomiting, rashes, petechiae, hematuria, and melena), laboratory findings (leucopenia < 3000/cm3), leukocytosis >9000 cm³), thrombocytopenia, abnormal Thromboplastin Time (PT) and Partial Thromboplastin Time (PTT), increased transaminase, aspartate aminotransferase (AST) and alanine transaminase (ALT) and the scores (if above 12). Therefore, the patient was suspected to have been infected with the CCHF virus and tested positive for the disease. Also, according to the guidelines, the patient had a probable case and was immediately treated with Ribavirin [2200 mg/kg Stat, then 1100 mg/kg (every 24 hours) for 4 days, 600 mg/kg (every 28 hours) for 6 days]. Her blood sample was taken and referred to the Pasture Institute in Iran for clinical examination involving a direct viral test (RT-PCR) and serological test (IgM), where she was put in isolation. Unfortunately, she died on 21st June 2020 due to a deteriorating condition and was buried under strict hygienic conditions.
| Parameters (Normal Limits) | 20th June, 2020 | 21st June, 2020 |
|---|---|---|
| Hb concentration g/dL (11.8 - 15.2) | 13.8 | 6 |
| Leucocytes/mm3 (4100 - 11300) | 11.2 | 5 |
| Thrombocytes cells/mm3 (150000 - 450000) | 10 | 12 |
| AST U/L (8 - 46) | 965 | 624 |
| ALT U/L (7 - 47) | 426 | 192 |
| PT s (11 - 15) | 35.5 | - |
| aPTT s (30 - 46) | >120 | 110 |
| Hct (36 - 46) | 41 | 17.5 |
| M.C.V fl (77 - 97) | 94.7 | 92.1 |
| Alp U/L (64 - 306) | 824 | 480 |
| Albumin g/dL (3.5 - 5.5) | 3.3 | 3 |
Abbreviations: Hb, haemoglobin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PT, prothrombin time; aPTT, activated partial thromboplastin time.
3. Discussion
CCHF, a tick-borne disease, is endemic in countries in Africa, southeastern Europe, Asia, and the Middle East; the CCHF virus has been reported in over 30 countries (14, 15). The CCHF virus causes serious outbreaks of viral hemorrhagic fever and has a 10 to 40% fatality rate (2). The current report on the 32-year-old housekeeper from the Kermanshah province in Iran confirms the fatality potential of CCHF and should be given more serious attention. Considering that the patient was a housekeeper who kept sheep, which are known hosts of the CCHF virus (10), it may be right to assume that the lady had picked up the CCHF virus through the bites of CCHF-infested ticks or by contact with infected sheep’s blood or tissues during and after slaughter. According to the World Health Organization (WHO), there is also the possibility of human-to-human transmission through close contact with blood, secretions, organs, or other body fluids from infected people (2). As stated by Ozaras et al. (15), those who live and work in environments where people are in close contact with animals and livestock enhance the risk of transmitting CCHF. Therefore, even though our report did not mention whether or not the patient lived in close contact with other people in her place of residence, there is the possibility of her getting infected by an already-infected person, perhaps due to her close contact with them. The fact that the patient never traveled abroad suggests that she might have been infected with the virus from her place of residence.
Our study is in contrast with a systematic review conducted in Turkey that reported that chances of co-infection were rare (16). In line with earlier research findings from different areas, the current results confirm that CCHF and COVID-19 have common clinical symptoms (17, 18) because our patient exhibited symptoms such as nausea, vomiting, fever, headache, and myalgia. The symptoms were accompanied by reduced platelet counts, decreased consciousness, and an INR value of above 6. This shows that the overlap of clinical manifestations of the two diseases can be confusing and has the potential to negatively affect the diagnosis and management of CCHF in particular. Indeed, the situation led to the initial diagnosis of our patient with COVID-19 when she actually had CCHF. Finding and reporting the epidemiological history of CCHF cases in the early stages is one of the most important approaches to the effective handling of the disease. The lack of knowledge about the symptoms of CCHF and its similarities with COVID-19 prevents patients from getting early referrals to physicians for diagnosis and treatment (18). This is, however, a cause of worry because, as reported in this study, even though our patient was immediately given CCHF treatment, she died after her condition deteriorated. Given that CCHF causes devastating outbreaks, a quick and accurate diagnosis is needed to effectively manage cases.
In conclusion, the current case of CCHF has serious and important public health implications for the people of Kermanshah province and Iran as a whole. Considering the frequent reports of the disease in Iran and the neighboring countries, coupled with the overlapping clinical features between the COVID-19 pandemic and CCHF, there is a need to consider differential diagnoses of patients who show COVID-19 symptoms in order to prevent misdiagnosis, undue delay in treatment and death. Though there is evidence to suggest that some healthcare systems have been involved in the management of COVID-19 and have no desire to engage in cases of other infectious diseases (19), it is advised that during pandemics, other infections that share similar clinical manifestations with COVID-19 (eg, CCHF) should be considered in patients with vague clinical symptoms, particularly in countries where other endemic diseases occur. Additionally, the disclosure of the patient's epidemiological history would enable early detection and effective diagnosis of CCHF. The capabilities of laboratories should also be strengthened so that they can safely detect and evaluate the CCHF virus. The W.H.O. should also liaise with the Iranian Ministry of Agriculture to organize programs to eradicate tick populations in CCHF-endemic areas. Finally, stakeholders should focus on educating individuals at higher risk of CCHF infection and supplying Personal Protective Equipment to those at lower risk.