1. Background
Coronavirus disease 2019 (COVID-19) has been characterized by unabated progression and increasing numbers of infections and deaths since its detection in December 2019 in China. According to the World Health Organization (WHO), as of October 17, 2022, there were 629,959,595 cases of COVID-19 infection worldwide, with 6,571,489 deaths. In the Republic of Kazakhstan, 1,394,287 cases have been registered, with 13,692 deaths. In our country, 959 people are still being treated for the infection, including 107 inpatients and 852 outpatients. A total of 38149 cases of COVID-19 infection were registered in Shymkent city in 2020 - 2022, including 1,646 pregnant women: 537 in 2020, 892 in 2021, and 217 in 2022 (1, 2). Concerning the pathogenicity, SARS-CoV-2 enters the target cell via surface receptors and the serine protease TMPRSS2, located on cells of various organs and tissues. The upper respiratory and digestive tracts are the entry gates for infection, where receptors for SARS-CoV-2 (ACE2 and CD147) and TMPRSS are located (3). According to a Moore et al. report, 15% had a severe course of infection, 5% had a critical course, and 80% had a mild to moderate course, which, according to the WHO, approximates the population figures (4).
Reduced immune reactivity and other physiological changes in the gestational period can increase susceptibility to respiratory disease and severe pneumonia in pregnant women, leading to admission to intensive care units and ventilatory support (5). As known, SARS-CoV-2-induced disease depends directly on virus entry into host cells after binding to angiotensin-converting enzyme 2. Angiotensin-converting enzyme 2 (ACE2) proliferates on cell membranes and is tropic in the placenta throughout gestation. This phenomenon is a possible etiology of the susceptibility of pregnant women to COVID-19 (6). Also, COVID-19 in pregnant women is severe due to the gestation process and the lack of standard therapy for the disease. Since August 2021, the etiotropic drug remdesivir has been added to the prescription list for women during gestation, with an intravenous regimen of 200 mg on day 1, followed by 100 mg daily for a five-day course. Remdesivir is an antiviral agent that blocks the reproduction of SARS-CoV-2 (7). This drug is recommended for use based on international experience and the results obtained on a group of adult patients by scientists in the Republic of Kazakhstan (8). The antiviral drug is approved for the etiotropic treatment of coronavirus infection in pregnant women by the decision of a medical consilium, provided that the potential benefit of the drug exceeds the potential risk to the patient (7).
2. Objectives
Currently, the global scientific community has limited publications on the efficacy of remdesivir in pregnant women with COVID-19. In the Republic of Kazakhstan, there are only sporadic studies on drug use in adults. However, there are no studies conducted on women during gestation. To assess the efficacy of remdesivir in female patients, we investigated the following criteria: Timing of temperature normalization, improvement in respiratory rate, and subjective reduction in dyspnea.
3. Methods
To carry out the study, 120 pregnant women with severe and extremely severe coronavirus infections, who were admitted to the Shymkent City Infectious Disease Center in 2021 - 2022, were subjected to comprehensive evaluation. The diagnosis of COVID-19 in pregnant women was made based on the epidemiological history, the complex clinical symptoms of the disease, and the results of laboratory and instrumental investigations. Inclusion criteria included confirmed and probable cases of coronavirus infection among pregnant women, severe and extremely severe course of COVID-19, and remdesivir use as etiotropic treatment. Exclusion criteria were pregnancy without COVID-19 and mild to moderate disease in pregnant women. We enrolled 120 out of 411 patients who fulfilled these criteria. Sample sizes were determined considering the confidence level of 95% and the test power of 80%. The sample size was calculated as 120 using G power software (version 3.1).
The patients were divided into two groups. The study group included women who received remdesivir at a dose of 200 mg intravenously on day one and standard therapy according to the clinical protocol for the diagnosis and treatment of coronavirus infection in pregnancy. The number of pregnant women was 60 women. The control group consisted of patients who received standard therapy per the above protocol. Sixty women were included in this group.
The following parameters were studied: Age, gestational age, delivery, and diagnosis by PCR. We took the following criteria for the efficacy of remdesivir therapy in pregnant women with COVID-19: Timing of temperature normalization, improvement in respiratory rate, and subjective reduction in dyspnea.
The Local Ethics Committee of the South Kazakhstan Academy approved the study protocol. Statistical analysis was performed using IBM SPSS Statistics 26.0 software. Nominal variables were analyzed using the Mann-Whitney, Pearson’s chi-square, and Fisher’s exact tests.
4. Results
Two groups of patients were studied. The study group included women who received remdesivir at 200 mg intravenously on the first day in addition to standard therapy according to the clinical protocol for the diagnosis and treatment of coronavirus infection in pregnant women. The control group (2) included patients who received standard therapy per the above protocol. There were 60 women in each group.
The following parameters were studied: Age, gestational age, delivery, and diagnosis by PCR. The results are presented in Table 1.
| Index | Absolute Number (%) | P-Value | |
|---|---|---|---|
| Therapy with Remdesivir (N = 60) | Therapy Without Remdesivir (N = 60) | ||
| Age, y | 0.019 a | ||
| 18 - 25 | 17 (28.3) | 22 (36.7) | |
| 26 - 32 | 18 (30.0) | 27 (45.0) | |
| 33 - 42 | 25 (41.7) | 11 (18.3) | |
| Gestational age, weeks | 0.537 | ||
| 1 - 12 | 1 (1.7) | 3 (5.0) | |
| 13 - 27 | 21 (35.0) | 18 (30.0) | |
| 28 - 40 | 38 (63.3) | 39 (65.0) | |
| Pregnancy parity | 0.111 | ||
| 1 | 6 (10.0) | 17 (28.3) | |
| 2 | 14 (23.3) | 8 (13.3) | |
| 3 | 14 (23.3) | 12 (20.0) | |
| 4 | 11 (18.3) | 8 (13.3) | |
| 5 and more | 15 (25.0) | 15 (25.0) | |
a Differences are statistically significant (P < 0.05).
Statistically significant differences were observed in age between the main and control groups (P = 0.019). The differences detected were due to a higher frequency in the age group of 33 to 42 years in patients taking remdesivir than in the control group (P = 0.036). The association between the compared features was medium (V = 0.250). Women aged 33 - 42 were 41.7% (n = 25) in the main group and 18.3% (n = 11) in the control group. Pregnancy term and parity were not statistically significant (P > 0.05). Most women were hospitalized with severe and extremely severe COVID-19 in the third trimester of pregnancy (63.3% and 65.0%, respectively). Pregnancy parity analysis showed that the most frequent indication for remdesivir was five or more pregnancies (25.0%; n = 15), followed by four or more pregnancies, 18.3% (n = 11), confirming the more severe course of COVID-19 in multiparous women. In the control group, primiparous women accounted for 28.3% (17).
Pregnant women with confirmed (U 07.1) and probable (U 07.2) coronavirus infection were included in the study. In the main group, 80% (n = 48) of the sample had been confirmed in the case group versus 90% (n = 54) in the control group. Probable cases of coronavirus infection were twice as common in the study group compared with controls (20%, n = 12 vs. 10%, n = 6, respectively). Comparing the frequency of diagnosis by PCR depending on the prescription of remdesivir showed statistically significant differences (P = 0.02). The likelihood of prescribing remdesivir increased by 0.44 in pregnant women with confirmed coronavirus infection (95% CI: 0.15 - 1.28).
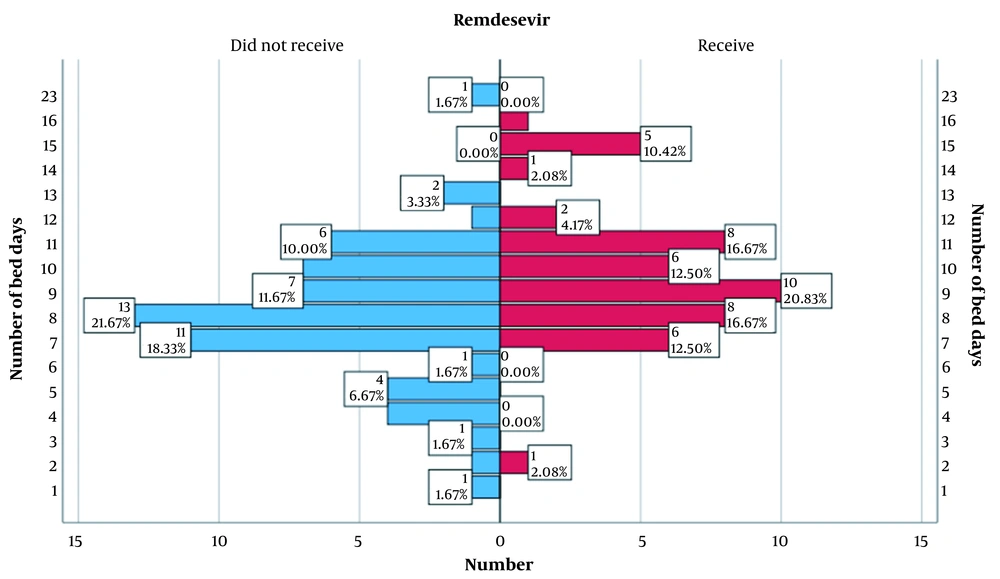
One of the indicators on which women during gestation were studied was the number of bed days in the hospital, as shown in Figure 1.
When comparing the main and control groups, there were statistically significant differences in bed days (P = 0.001). Women in the core group (Median = 9.00; Q1 - Q3 = 8.00 - 11.0) stayed longer in the hospital than the control group (Mo = 8.00; Q1 - Q3 = 7.00 - 10.0). This is due to the more severe condition of patients in this group.
The results were not statistically significant (P > 0.05) (Table 2). However, there were 14 cases of preterm births (8 in the main group and 6 in the control group). More than half of the pregnancy complications were due to placenta detachment: 53.3% in the main group and 50% in the control group. There was one case of intrauterine fetal death in the control group. There were 6 cases of postpartum hemorrhage during the study period (60% in the first group and 75% in the second group) and one case of sepsis in the control group. The fatal case occurred in the control group (Figure 2).
| Index | Absolute Number (%) | P-Value | |
|---|---|---|---|
| Therapy with Remdesivir (N = 60) | Therapy Without Remdesivir (N = 60) | ||
| Outcome of pregnancy | 0.795 | ||
| Preterm birth | 8 (13.3) | 6 (10.0) | |
| Prolongation | 36 (60.0) | 40 (66.7) | |
| Birth at term | 15 (25.0) | 14 (23.3) | |
| Complication of pregnancy | 1.000 | ||
| Intrauterine fetaldeath | 0 (0) | 1 (6.3) | |
| Placental abruption | 8 (53.3) | 8 (50.0) | |
| Pre-eclampsia | 7 (46.7) | 6 (37.5) | |
| Chorioamnionitis | 0 (0) | 1 (6.3) | |
| Complications in the postpartum period | 2.925 | ||
| Bleeding | 3 (60.0) | 3 (75.0) | |
| Sepsis | 0 (0) | 1 (25.0) | |
| Endometritis | 2 (40.0) | 0 (0) | |
| Outcome of treatment | 0.180 | ||
| Recovery | 1 (1.7) | 3 (5.0) | |
| Improvement | 59 (98.3) | 56 (93.3) | |
| No change | 0 (0) | 1 (1.7) | |
| Mortality | 0 (0) | 0 (0) | |
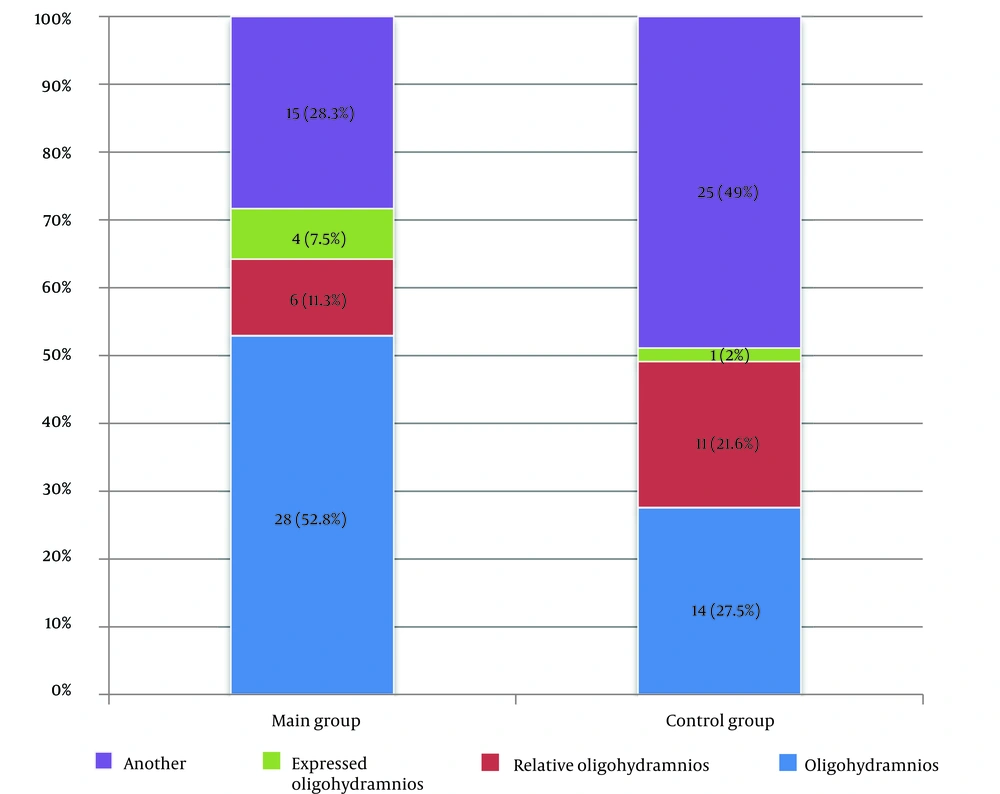
There were statistically significant differences in amniotic fluid changes according to ultrasound between the control and main groups (P = 0.013). When comparing the groups in pairs, oligohydramnios was more common in women who received Remdesivir (P = 0.316) than in the control group. Oligohydramnios was detected in 52.8% (n = 28) of cases in the main group and 27.5% (n = 14) in the control group.
We took the following criteria for the efficacy of Remdesivir therapy in pregnant women with COVID-19: Timing of temperature normalization, improvement in respiratory rate, and subjective reduction in breathlessness. The normalization of body temperature on day 1 or 2 occurred in 68% (n = 41) of pregnant women in the control group, associated with a less severe course of COVID-19 in this group of patients, whereas in the main group, it occurred in 28% (n = 17). Also, SpO2 increased by more than 95%, leading to the cancellation of oxygen therapy on days 1 - 2 in the main group in 71% (n = 43), before four days of antiviral therapy in 10% (n=6), and days 7 - 8 after the start of etiotropic treatment only in 68% (n = 41). In the other group, cancellation of oxygen therapy due to improvement in SpO2 by more than 95% was observed in 26 pregnant women (43%) on day 1, 38% (n = 23) on days 3 - 4, 15% (n = 9) on days 5 - 6, and 4% (n = 2) on days 7 - 8 after the start of therapy.
The respiratory rate dynamics were as follows: In the study group, 22 (36.6%) women had normalization of respiratory rate on the third day after the start of Remdesivir therapy, 25 (41.6%) had it later than three days after the start of therapy. In the remdesivir therapy group, the subjective sensation of dyspnea in pregnant women stopped on day 2 in four (6.6%) patients, day 3 in 11 (18.3%), and later in 40 (66.6%) patients.
5. Discussion
Various researchers have reported that gestational women with coronavirus infection are more prone to progress to severe respiratory disease than non-pregnant women (9, 10). The etiologies are a weakened immune system, an increased risk of thromboembolism, and increased concentrations of circulating pro-inflammatory mediators (10, 11). Coronavirus infection during pregnancy can also lead to pre-eclampsia, preterm labor, and stillbirth, most commonly in patients with severe COVID-19 (10, 11). The difficulty in the clinical management and drug treatment of pregnant women with COVID-19 is related to the risk of possible teratogenic effects of the drugs (10, 11). During the COVID-19 pandemic, many drugs were prescribed without evidence of efficacy and guaranteeing no long-term effects on the fetus (10). The development of etiotropic drugs was a process that took several years. Drug development was based on a study of the efficacy of existing antiviral drugs that could be effective against SARS-CoV-2 (12-15). Remdesivir was administered as a solution. To date, there are a limited number of studies evaluating the efficacy of remdesivir in pregnant women with COVID-19 worldwide. Meanwhile, there are no publications in this cohort in our country. There are only sporadic studies on the use of the drug in adults (12). Therefore, this issue is relevant and requires further study. Early positive results of this antiviral agent led to its emergency use in pregnant women with COVID-19 (16-20). Further studies are needed to evaluate its efficacy and safety in patients with COVID-19 so that it can be recommended in international COVID-19 treatment protocols (13-17, 21).
Women in the 33 - 42 age group (41.7%) received antiviral therapy more often than other pregnant women in the younger age group (30.0% and 28.3%). The third trimester was marked by hospital admission among patients. A more severe course of coronavirus infection was observed in multiparous mothers. The duration of hospital stay was longer in the main group of pregnant women (9.0 bed days) than in the control group (7.0 bed days). Amniotic fluid examination showed that scant amniotic fluid was more common in the study group than in the control group (52.8% and 27.5%, respectively). The criteria for the efficacy of remdesivir in women with COVID-19 during gestation were based on the dynamics of temperature normalization, improvement in respiratory rate, and subjective reduction in breathlessness. Our study found that a decrease in temperature to normal levels occurred earlier in the control group (68%) than in the main group. A further increase in SpO2 by more than 95% was seen on days 3 - 4 in the main group (71%) and on days 1 - 2 in the control group (43%). After three days, there was an improvement in respiratory rate (41.6%) and a reduction in subjective dyspnea (66.6%) in the main group.
A Randomized Controlled Trial (RCT) showed that remdesivir therapy in pregnant women with Ebola was safe and without significant side effects (22). The use of this antiviral drug for moderate to severe COVID-19 requiring oxygenation has shown modest benefit (23). This was also confirmed in our study. According to various studies, among pregnant women with SARS-CoV-2 infection, the use of remdesivir for five days leads to a clinical improvement in the course of moderate COVID-19 (24), which is consistent with data in the general population (25). Burwick et al. reported that remdesivir given intravenously for 10 days clinically improves severe COVID-19 in pregnant and postpartum patients (26). But in our study, the use of a 5-day course of remdesivir showed no clinical improvement in patients.
Recruitment of pregnant women with COVID-19 during the pandemic has been rapid, which is one of the study’s strengths. However, the limitation was the creation of a comparison group. The prescription list for patients with severe and extremely severe COVID-19 included the antiviral drug remdesivir. Therefore, the comparison group included those pregnant women who did not give informed consent to additional treatment. Another limitation was that this study is a single center, which does not provide extended results. It should be noted that the sample size was relatively small.
5.1. Conclusions
A more severe course of coronavirus infection occurs in second-born women. Older age and third-trimester pregnancy are risk factors for progression to severe disease. The etiotropic agent remdesivir is not of reliable efficacy. There are a limited number of studies worldwide on using remdesivir in pregnant women with COVID-19. Further studies are needed to evaluate its efficacy and safety in gestational patients with COVID-19 for recommendations in international treatment protocols for coronavirus infection.