1. Background
The current pandemic of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to a significant mortality rate worldwide during the past years. Based on the World Health Organization (WHO) statistics, by the current date (May 3, 2023), there are more than 765 million confirmed cases and 6.9 million coronavirus disease 2019 (COVID-19)-associated deaths. Iran’s official statistics reported 7,607,744 confirmed cases and 146,058 deaths by that date (1). With the surge of the delta variants of the SARS-CoV-2, COVID-19 statistics have risen significantly worldwide and in Iran (2). The efficacy of different vaccine platforms in facing different virus variants and mechanisms of virus escape from neutralization antibodies are currently significant challenges (3, 4).
Meanwhile, many vaccination cases in different countries have reduced COVID-19 mortality, and different countries use different vaccines for their populations (5). Currently, there are different approved and emergency-approved vaccines by WHO (6). One of the promising and available vaccines from this list in Iran is Sputnik V (Gam-COVID-Vac).
The Gam-COVID-Vac is an adenoviral-based vector vaccine platform. The vaccine was generated based on two different adenovirus vectors: Recombinant adenovirus (rAd) 26 and rAd 5 (7). A phase III study represented high primary efficacy (91%) for Sputnik V (7). The safety profile of this vaccine is represented by several studies (7-9). Regarding efficacy, some studies have suggested that a single dose of Sputnik V could provoke a high antibody level in vaccine recipients with a history of COVID-19 infection (10, 11). The Gam-COVID-Vac (Sputnik V) COVID-19 vaccine is an approved vaccine in Iran.
2. Objectives
In the current retrospective study, we evaluated the Sputnik V COVID-19 vaccine's short-term efficacy and side effects in 65 Iranian healthcare providers receiving Sputnik V.
3. Methods
3.1. Participants and Vaccination
The current study evaluated the Sputnik V antibody levels and side effects in 65 healthcare providers from Firoozgar Hospital affiliated with the Iran University of Medical Sciences, Tehran, Iran, between March 2021 and December 2021. The participants were randomly selected using no particular demographical criteria for the selection. The sample size was determined based on the personnel referred to the hospital for vaccination, the study period, and limited to the study period, and the participants were randomly selected based on the cases referred to the clinic. Written ethical consent was obtained at the onset of the follow-up under the ethics code IR.IUMS.FMD.REC.1400.204. Included participants were healthcare providers aged more than 18 years who received two doses of the COVID-19 vaccine and signed the informed consent. Patients with current COVID-19 and those who still need to complete questionnaires or continue the follow-up were excluded. The vaccination was performed according to the manufacturer's guideline for all participants, including a first dose (1 cc) and the second dose (1 cc) at a 21-day interval. Intramuscular injection (the preferred site is the deltoid muscle) was done with a single dose of the vaccine (each dose contains 1cc of water-soluble lyophilized vaccine). The vaccination was performed from April to May 2021. Based on the local epidemiological studies, the delta variant was the dominant variant in the evaluated geographical location during that particular time.
3.2. Sampling and Antibody Evaluation
After the first and the second doses of the vaccination, mild side effects were recorded using a checklist, including headache, fever, myalgia, pain in the injection site, diarrhea, nausea, and vomiting based on the participants’ complaints. Humoral immunity against S-RBD IgG of SARS-CoV2 after Sputnik V vaccination was evaluated in three phases, including phase I on day 60 (one month after the second dose), phase II on day 120 (three months after the second dose), and phase III on day 210 (six months after the second dose). Thus, one month, three months, and six months after the second vaccination dose, 5 mL of the peripheral blood was collected from all participants for the antibody assessment. The serum samples were used for the antibody evaluation via the enzyme-linked immunosorbent assay (ELISA) method and the anti-S IgG ELISA kit (Hayan Pajooh Pars, Iran). Chemobind ELISA kit (Hayan Pajouh Pars, Iran) employs indirect enzyme-linked immune-sorbent technology. The ELISA was performed according to the manufacturer's protocols. The ELISA results were reported as the ratio, and the cut-off value for the ELISA was considered 1.1 as negative and equal to or more than 1.1 as positive. The antibody concentration was reported based on the optical density (OD) value of each sample. The diagnostic specificity of the Chemobind ELISA kit (Hayan Pajouh Pars, Iran) was defined as 100%, and its diagnostic sensitivity was 100%.
3.3. Statistical Analysis
The statistical analyses were performed using SPSS version 22 (IBM, Chicago, USA), and the P-values < 0.05 were considered significant. The chi-square or Fisher’s exact test for categorical data and the student t-test for normal data were run to evaluate the associations between different variables.
4. Results
4.1. Demographical Results
We evaluated the Sputnik V antibody levels and side effects in 65 vaccine recipients. The mean age of the vaccinated population was 35 ± 8.5 years. In addition, we had 27 male (41.5%) and 38 female (58.5%) participants. Before the vaccination, 41.5% of vaccine recipients had a history of infection with SARS-Cov2. COVID-19 resulted in a mild symptomatic infection in all participants except for one, which led to hospitalization. Evaluation of the comorbidities in the vaccinated population revealed only one case of type 2 diabetes.
4.2. Antibody Levels After the Second Dose
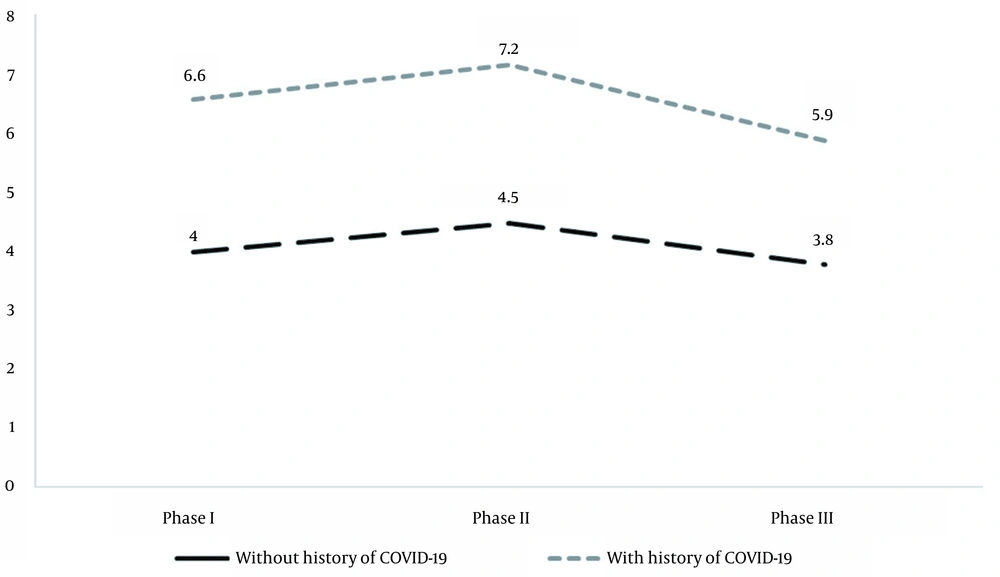
The antibody evaluation using the ELISA was performed one month, three months, and 6 months after receiving the second dose. Figure 1 shows the OD levels of the SARS-CoV-2 IgG antibody in recipients with no confirmed history of SARS-CoV-2 infection. The OD levels for SARS-CoV-2 IgG were higher in recipients with a history of COVID-19 at each time point (P = 0.001). Also, the antibody levels dropped after six months in both groups of vaccine recipients. The mean IgG levels one month after vaccination was 4 ug/dL for participants without a history of COVID-19 infection and 6.6 ug/dl for those with previous infections. This value was 4.5 ug/dL and 7.2 ug/dL three months after vaccination and 3.8 ug/dL and 5.9 ug/dL six months after vaccination, respectively.
Mean optical density (OD) of the SARS-CoV-2 IgG in vaccine recipients with and without a history of COVID-19 in phase I on day 60 (one month after the second dose), phase II on day 120 (three months after the second dose) and phase III on day 210 (six months after the second dose) of Sputnik V vaccination.
4.3. Vaccination Side Effects
The major complication after the first and second doses of vaccination was myalgia (33.8% and 27.7%, respectively). Also, after the first dose of the vaccination, some rare side effects, like vomiting, were seen in 1.5% of the recipients. More information on the side effects of the vaccination after the first and the second doses is presented in Table 1. There was no significant difference in side effects after the first and the second doses of vaccination between vaccine recipients with and without a history of COVID-19, except for headaches after the second dose. After the second dose, the headache was reported in four vaccine recipients with a history of COVID-19, while no patient without a history of COVID-19 had this complaint (P = 0.034). Among all 65 vaccine recipients, only one was admitted to close monitoring for 3 days after the first dose.
| Vaccine Dose and Side Effect | Total | No. (%) | P-Value | ||
|---|---|---|---|---|---|
| No. (%) | P-Value | With COVID-19 History | Without COVID-19 History | ||
| First dose | |||||
| Headache | 6 (9.2) | 0.32 | 4 (33.3) | 2 (66.7) | 0.41 |
| Fever | 14 (21.5) | 0.54 | 8 (57.1) | 6 (42.9) | 0.54 |
| Myalgia | 22 (33.8) | 0.5 | 10 (45.5) | 12 (54.5) | 0.99 |
| Pain in the injection site | 2 (3.1) | 0.13 | 0 (00.0) | 2 (100.0) | 0.22 |
| Diarrhea | 2 (3.1) | 0.56 | 1 (50.0) | 1 (50.0) | 0.99 |
| Nausea | 18 (27) | 0.43 | 10 (55.6) | 8 (44.4) | 0.58 |
| Vomiting | 1 (1.5) | 0.34 | 0 (00.0) | 1 (100.0) | 0.99 |
| Second dose | |||||
| Headache | 4 (6.2) | 0.034 a | 4 (100.0) | 0 (00.0) | 0.04 |
| Fever | 9 (13.8) | 0.22 | 6 (66.7) | 3 (33.3) | 0.29 |
| Myalgia | 18 (27.7) | 0.71 | 8 (44.4) | 10 (55.6) | 0.78 |
| Pain in the injection site | 1 (1.5) | 0.49 | 1 (100.0) | 0(100.0) | 0.47 |
| Diarrhea | 0 (0) | 0.5 | 0 (00.0) | 0 (00.0) | - |
| Nausea | 9 (13.8) | 0.61 | 5 55.6) | 4 (44.4) | 0.72 |
| Vomiting | 2 (3.1) | 0.47 | 0 (00.0) | 2 (100.0) | 0.49 |
a Represents the statistically significant difference between the vaccinated population with and without a history of COVID-19.
4.4. COVID-19 Infection After the Vaccination
By the 6-month follow-up of all 65 participants, we found that 18 cases (27.7%) were infected with SARS-CoV2. All these participants were from the group without a history of COVID-19, with a mean age of 37.9 ± 8.5 years, and 11 cases (61%) were females. There were no statistically significant associations between demographical data and different side effects in participants infected with SARS-CoV2. All these 18 cases had a mild infection, except for one, and all survived. Neither mean antibody levels one month after vaccination (P = 0.01) nor at 3- or 6-months post-vaccination were significantly lower in patients infected with SARS-CoV2 after vaccination with the Sputnik V vaccine.
5. Discussion
In the current study, using ELISA, antibody evaluation was performed for one month (phase I on day 60), three months (phase II on day 120), and 6 months (phase III on day 210) after receiving the second dose of the Sputnik V vaccine. The mean OD levels for SARS-CoV-2 IgG were statistically higher in recipients with a history of COVID-19 (P = 0.001). The antibody levels dropped after 6 months in both groups of vaccine recipients. Logunov et al. reported phase III results of the Sputnik vaccine results. The results proved promising, with 91% primary efficacy and high neutralization antibody titer in vaccine recipients (7). It has even been reported that a single dose of Sputnik V can significantly increase the antibody level in vaccine recipients without a history of COVID-19 (10). Also, the induction of memory B cell responses in Sputnik V was promising (12). Regardless of the high efficacy of the Sputnik V against the wild-type SARS-CoV-2 and the alpha variant (VOC 1, B.1.1.7), there are some concerns about the efficacy of the vaccine in dealing with the emerged variants, such as beta or B.1.351 (13). This concern could be considered a further challenge for all variants harboring the E484K substitution, especially the delta variant (B. 1.617). In this regard, Gushchin et al. reported that the Sputnik V neutralization activity is not significantly different between any particular VOCs, including beta, gamma, delta, and the reported geographically specific variants of Russia (B.1.1.141 and B.1.1.317) (14). In addition, another study in Argentina reported higher antibody levels in the COVID-19 pre-vaccination period (15). Although our knowledge of the efficacy of Sputnik V against different variants is incomplete, the high efficacy of the Sputnik V vaccine and greater response to the vaccine in vaccinated people with a history of COVID-19 is clear. Our study reported a drop in antibody levels after 6 months in both groups of vaccine recipients. Likewise, a drop in antibody levels after 6 months was reported in a study on 602 healthcare workers in Argentina (15). The findings of the current study support the previous studies in this field. However, the importance of further studies to evaluate the Sputnik V vaccine efficacy in facing different variants of SARS-CoV-2 is warranted.
The major side effect observed after the first and the second doses of the vaccine in the current study was myalgia. There was no significant difference in side effects after the first and the second doses of vaccination between vaccine recipients with and without a history of COVID-19, except for headaches after the second dose. In a study by Babamahmoodi et al., the efficacy and side effects of the Sputnik V were evaluated in 13435 Iranian healthcare workers. As reported, pain in the injection site, fatigue, myalgia, headache (35.7%), fever, and joint pain were the most frequent side effects, respectively (16). In the study by Razazian et al. (17) in Iran, the most common adverse effects after the first dose of COVID-19 vaccination were fatigue (30.1%), myalgia (29.8%), fever (25.0%), and headache (22.3%), and fatigue (27.1%), headache (18.6%), myalgia (17.5%) and fever (14.9%) after the second dose. However, 188 patients with multiple sclerosis (MS) were studied. Another study was conducted in Iran by Babaee et al. (18) on 1751 (36.7%) participants who received the Sputnik V vaccine. They reported adverse effects in 82.7% of participants, and the most common complications included fatigue, skeletal/muscle pains, chill/fever, injection site reactions, and headache. Headache after the second dose of the vaccine was reported to be more frequent in the study by Montalti et al. (8), but this difference was not statistically significant. According to the studies, no blood clotting or any particular severe and critical adverse effects have been reported in Sputnik V recipients (8, 9). Montalti et al. (8) reported that pain at the injection site was the most common side effect in 24% of the recipients after the first dose and in 48% of the recipients after the second dose, followed by asthenia, headache, and joint pain. Also, Pagotto et al. (9) reported injection site reactions as the most common side effect. Myalgia (68%), fever (40%), and diarrhea (5%) were reported as other important adverse events after the Sputnik V vaccination in Italy. A pre-trained deep learning algorithm for the evaluation of the Sputnik V with an open participatory trial in Telegram by Jarynowski et al. revealed pain (47%), fever (47%), fatigue (34%), and headache (25%) as major complains in vaccine recipients (19).
The differences between the reported side effects of this vaccine seem to be due to the small number of evaluated samples in our study, which is our major limitation. As another limitation of the current study, we could not use other validated methods, like immunofluorescence or western blot, to support and approve antibody production or quantity.
After 6 months, we found that 18 vaccinated participants (27.7%) contracted SARS-CoV2. None of these individuals had a history of COVID-19 before vaccination. Several studies have reported the vaccination breakthrough in different countries and vaccine platforms (20-23). Meanwhile, there is no report of particular age or gender differences in the population with vaccination breakthroughs (20). Bergwerk et al. demonstrated the importance of antibody levels after vaccination and vaccine breakthroughs (24). It seems that the results of our study are in consensus with those of the previous studies using other vaccine platforms.
There were some drawbacks in our study. The first was the limited sample size due to the study enrollment in one center and limited study duration. Participants were followed up three times, another factor affecting our sampling. Another limitation was the study of other SARS-CoV2 markers, such as Spike or RBD IgM, to find the asymptomatic infections, which possibly affected our participant classifications and the interpretation of results. Current COVID-19 infection or the history of infection or unknown elevated antibody levels could be found by the measurement of baseline SARS-CoV2 antibody levels for more comprehensive interpretations, which was another limitation of our present study.
5.1. Conclusions
In conclusion, the present study presents Sputnik V as an acceptable vaccine based on the induced antibody levels after the second dose in both patients with and without a history of COVID-19. However, high levels of antibody secretion were obtained three months after the second dose of vaccination, and the decrease in antibody levels after six months indicated the relatively short duration of its immunogenicity after two doses of vaccination and the possible need for a booster; nonetheless, further investigations are needed.