1. Context
By definition, community-acquired pneumonia (CAP) is an acute pulmonary parenchymal infection acquired in patients outside a hospital setting or admitted for less than 48 hours, which can lead to considerable mortality, morbidity, and cost (1). It has a broad spectrum of clinical presentations from mild to severe pneumonia, particularly in older age and major comorbidities. Even though clinical management has improved over the last decades, the overall CAP incidence remains high, and its annual rate is estimated at around 16 to 23 cases per 1000 persons (2-4). Also, the pneumonia rate gradually increases with age > 50 (5), so the yearly incidence of hospitalization due to CAP among patients more than 65 years old is around 2000/105 in the United States (3, 6).
1.1. Risk Factors for Community-Acquired Pneumonia
Data from several studies defined dominant risk factors for CAP as older age, chronic comorbidities (chronic obstructive pulmonary disease, asthma, bronchiectasis, diabetes mellitus, immunocompromising conditions, malnutrition, and stroke), viral respiratory tract infection, smoking and alcohol misuse, and impaired airway protection (3, 7).
1.2. Microbiology
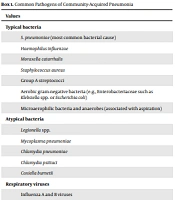
The most causative identified pathogens in CAP are Streptococcus pneumoniae (pneumococcus) and respiratory viruses. In addition, the common pathogens can be grouped into three categories (Box 1) (7, 8). Contrary to many detected pathogens in sputum samples, in a large number of cases, approximately 62% of executed studies in a hospital setting, there is no pathogen isolated (7, 9, 10).
| Values |
|---|
| Typical bacteria |
| S. pneumoniae (most common bacterial cause) |
| Haemophilus influenzae |
| Moraxella catarrhalis |
| Staphylococcus aureus |
| Group A streptococci |
| Aerobic gram-negative bacteria (e.g., Enterobacteriaceae such as Klebsiella spp. or Escherichia coli) |
| Microaerophilic bacteria and anaerobes (associated with aspiration) |
| Atypical bacteria |
| Legionella spp. |
| Mycoplasma pneumoniae |
| Chlamydia pneumoniae |
| Chlamydia psittaci |
| Coxiella burnetii |
| Respiratory viruses |
| Influenza A and B viruses |
| Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) |
| Other coronaviruses (e.g., CoV-229E, CoV-NL63, CoV-OC43, CoV-HKU1) |
| Rhinoviruses |
| Parainfluenza viruses |
| Adenoviruses |
| Respiratory syncytial virus |
| Human metapneumovirus |
| Human bocaviruses |
Common Pathogens of Community-Acquired Pneumonia
1.3. Scope and Purpose
According to published observational studies in Iran, this evidence-based guideline focuses on antimicrobial treatment and care standardization in the general population by providing management strategies. Regional guidelines are a need to evaluate and select the best available treatment due to local differences, etiologies, antibiotic susceptibility, drug licensing, healthcare infrastructure, and available resources. Taken together, the guidelines meet the healthcare professional need to select initial empiric antibiotic therapy and evaluate infectious disease management subsequently.
2. Methods
A multidisciplinary expert panel reviewed prospective, retrospective, analytical, and descriptive relevant reporting data published in IranMedex, Irandoc, MagIran, Google Scholar, Scopus, PubMed, SID (Scientific Information Database), and PubMed from January 1990 to August 2020. These reports focused on frequency, serotype distribution, and antimicrobial resistance patterns of circulating Streptococcus pneumoniae, Moraxella catarrhalis, Mycoplasma pneumonia, Haemophilus, and influenzae serotypes in Iran attained from clinical samples (e.g., ear, eye, nasopharynx, blood, cerebrospinal, pleural, joint or peritoneal fluids, or tracheal aspirates in clinical cases or nasopharyngeal specimens in carriers). In brief, the list of the most important causative pathogens of CAP was finalized in Box 1.
2.1. Grading of Guideline Recommendations
We followed the grading of recommendations, assessment, development, and evaluation (GRADE) criteria and expert opinion to assess the evidence for each recommendation. In this system, grades have two components: The two-level representation of the strength of the recommendation (strong or weak) and the four-level representation of the evidence certainty rating of high, moderate, low, and very low (11).
The judgment of the level of risks or benefits defines the strength of the recommendation. A weak recommendation is revealed either when risks and benefits are more closely balanced or are more unclear. A strong recommendation is that the benefits noticeably overshadow the risks (or vice versa) for roughly all patients (11).
Certainty of the evidence has a four-tier scale of evidence quality that reflects confidence in the approximations of benefits, harm, and burdens. This guideline uses A, B, C, and D letters to reflect high, moderate, low, and very low-quality evidence, respectively (11).
3. Recommendations
3.1. Outpatient Setting
See Figure 1.
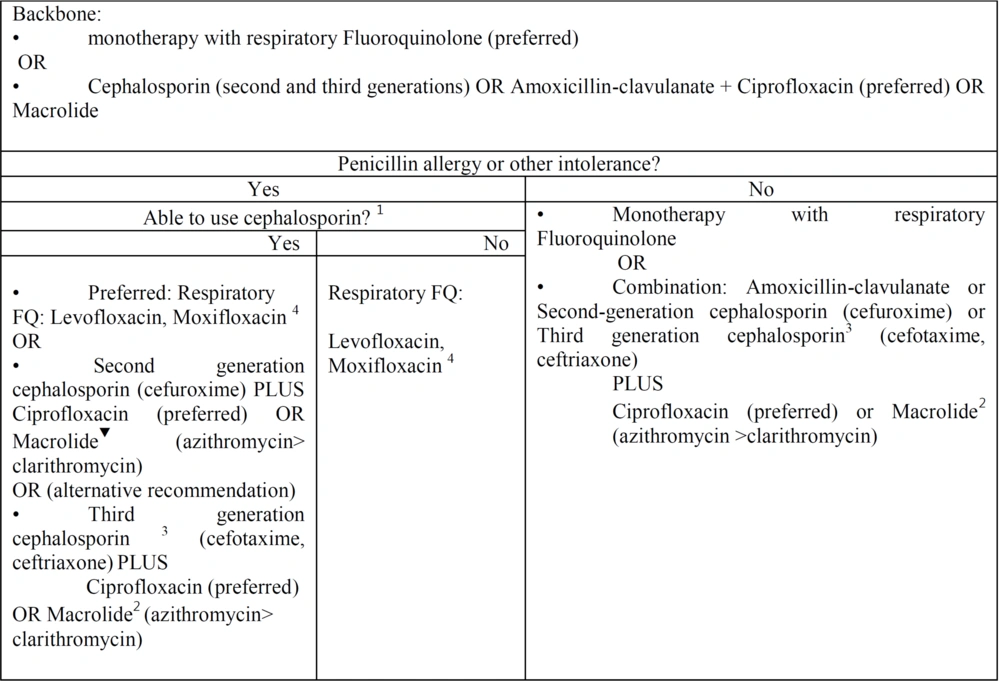
Outpatient community-acquired pneumonia. Empiric antibiotic selection in outpatient setting: This figure is adjusted of community-acquired pneumonia UpToDate and IDSA algorithms for treatment of outpatients according our published data and resistance antibiotic rate in Iran. 1 Patients with mild non-IgE-mediated reactions (eg, maculopapular rash) to penicillin or known cephalosporin tolerance can generally use later-generation cephalosporins safely. Patients with IgE-mediated reactions (hives, angioedema, anaphylaxis) or severe delayed reactions should generally use other agents. Refer to the UpToDate text on penicillin hypersensitivity reactions for detail. 2 Reasons to avoid macrolides include baseline prolonged QTc interval or risk for QTc prolongation (eg, hypokalemia, hypomagnesemia, clinically significant bradycardia, or use of other QT-prolonging agents). 3 If Second generation cephalosporin, as oral agents were not available, however monitoring and injection equipment were available in an outpatient clinic, we recommend third generation cephalosporin with close monitoring as an alternative regimen. 4 According to our data we recommend levofloxacin 500 or 750 mg daily due to lack of data about moxifloxacin 400 mg daily.
3.1.1. In Outpatient Settings, Which Empiric and Initial Antimicrobial Treatment Regimens Are Recommended for CAP in Adults?
For outpatient adults with Penicillin allergy or other intolerance:
If the patient can use cephalosporin, we recommend the following:
- Monotherapy: Preferred, strong recommendation, moderate quality of evidence; Respiratory FQ, levofloxacin, Moxifloxacin. According to our data, we recommend levofloxacin 500 or 750 mg daily due to a lack of data about moxifloxacin 400 mg daily.
Alternative (strong recommendation, moderate quality of evidence); second-generation cephalosporin (cefuroxime 500 mg twice daily). PLUS: Ciprofloxacin 500 mg every 12 hours (preferred antibiotic) or macrolide (500 mg azithromycin on the first day and 250 mg per day (preferred) or 500 mg clarithromycin twice daily). Alternative: If second-generation cephalosporin as oral agents was not available and monitoring and injection equipment were available (weak recommendation, high quality of evidence), third-generation cephalosporin (cefotaxime 1 - 2 g every 8 hours, ceftriaxone 1 - 2 g daily).
- PLUS: Ciprofloxacin 500 mg every 12 hours (preferred antibiotic) or macrolide (500 mg azithromycin on the first day and 250 mg per day (preferred) or 500 mg clarithromycin twice daily).
If patients are unable to use any cephalosporin, we recommend the following: (1) Monotherapy (strong recommendation, high quality of evidence); (2) respiratory fluoroquinolone (levofloxacin, moxifloxacin; according to our data, we recommend levofloxacin 500 or 750 mg daily due to a lack of data about moxifloxacin 400 mg daily).
For outpatient adults without penicillin allergy or other intolerance (strong recommendation, high quality of evidence):
- Monotherapy: According to our data, we recommend levofloxacin 500 or 750 mg daily and moxifloxacin 400 mg daily).
- Combination therapy: Strong recommendation, high quality of evidence, amoxicillin 500 mg/clavulanate 125 mg three times daily (or amoxicillin 875 mg and clavulanate 125 mg twice daily), or second-generation cephalosporin (cefuroxime 500 mg twice daily) or third-generation cephalosporin (cefotaxime 1 - 2 g every 8 hours, ceftriaxone 1 - 2 g daily).
- PLUS: Ciprofloxacin 500 mg every 12 hours (preferred antibiotic) or macrolide (500 mg azithromycin on the first day and 250 mg per day (preferred) or 500 mg clarithromycin twice daily). Summary of the evidence: The insufficient RCT data about antibiotic therapy for adults with CAP in Iran leads to administering inappropriate antibiotic regimens in patients. We recognized 94 relevant descriptive cross-sectional studies regarding antibiotic resistance for treating patients with CAP.
3.1.1.1. Summary of the Evidence
Cross-sectional studies regarding antimicrobial treatment regimens for patients with CAP offer evidence of the advantage of FQs over other antibiotic regimens. Based on these data, our recommendations for patients with or without comorbidities such as chronic heart failure, chronic lung, liver, or renal diseases, diabetes mellitus, alcoholism, neoplasms, or asplenia are similar due to antibiotic resistance in the Iran community, first-line treatment is monotherapy with respiratory FQ, based on the results demonstrating high antibiotic resistance to amoxicillin, amoxicillin-clavulanate, macrolides, and doxycycline.
The antibiotic resistance to amoxicillin-clavulanate for H. influenza is around 85.7% (12), although this index is controversial among M. catarrhalis samples. In some studies, the resistance rate was reported between 0% and 6.2% (13-15), whereas in another study, the maximum resistance rate was detected (16).
An amoxicillin-resistance pattern was reported in several studies. Two studies reported 100% and 81.2% rates for M. catarrhalis (13, 14). Also, approximately one-third of S. pneumoniae isolates had a resistance pattern (17), and increasing penicillin resistance was reported due to their extensive administration and recording mef (A) and erm (B) genes in 7.3 - 40% of cases (18).
According to published data in Iran, among third-generation cephalosporins, the fewest antibiotic resistance was related to ceftriaxone (6.2% for M. catarrhalis, 0%, 11.1%, and 28.6% for H. influenza) and cefotaxime (11.1% for H. influenza) against H. influenza, M. catarrhalis, and S. pneumoniae (12, 13, 19-21).
The mean percentage of macrolide resistance was estimated at 48.43% for S. pneumoniae. In detail, the least resistance rates were detected for azithromycin (16.4%), followed by clarithromycin (18.2%) and erythromycin (25.5%) by the E-Test (18, 22). In addition, the resistance rate for M. catarrhalis and H. influenza was reported as 0% - 28.6% (12-15, 20) in different studies. The overall pooled macrolide-resistant M. pneumoniae (MRMP) prevalence in the world was detected at 52% (38% - 65%) (19). As reported by subgroup analyses among 21 studies in Asia, the mentioned prevalence was estimated at 63% (52% - 75%), a critical dilemma in East Asia. Additionally, macrolide resistance was relatively high in Iran, and 56.9% of M. pneumoniae (MP) was detected in 270 specimens rated as MR-MP. The most isolated types in this study were 3/5/6/2 and 4/5/7/2, which significantly correlated with macrolide resistance (23).
The antibiotic resistance rates of ciprofloxacin and levofloxacin as anti-pseudomonal FQs were evaluated in different studies. Ciprofloxacin had 0% - 11% for S. pneumoniae, 0% and 70% for M. catarrhalis, and 0% and 57.1% for H. influenza (12, 13, 15, 19, 21). Likewise, levofloxacin was sensitive against M. catarrhalis and H. influenza with a 0% resistance rate (14, 21).
The antibiotic resistance rate against levofloxacin for respiratory FQ was estimated at 0% for both M. catarrhalis and H. influenza (12, 14, 21). Also, the resistance rate of levofloxacin in Iranian children according to a subgroup analysis of 27 studies and the total frequency resistance rate was reported as 0.8% and 1.7% for S. pneumoniae, respectively (24).
For MP, the first-line treatment is Macrolides, tetracyclines, and fluoroquinolones, and the decreased resistance rate of Macrolide-resistant MP (MR-MP) strains occurred by replacement of macrolides with tosufloxacin as a fluoroquinolone in Japan. As a result, although there are not enough data about fluoroquinolone resistance, these antibiotics could be considered an appropriate alternative to MR-MP (25-27).
3.2. Inpatient Settings
3.2.1. In Inpatient Settings, Which Empiric and Initial Treatment Regimens Are Recommended for CAP in Adults?
For hospitalized patients, the frequently identified pathogens are enteric gram-negative bacilli, S. aureus, S. pneumoniae, H. influenzae, M. catarrhalis, and atypical pathogens, including MP, Legionella spp., and respiratory viruses (e.g., influenza, parainfluenza, RSV, rhinovirus).
3.2.2. In Patients with CAP Without Risk Factors for MRSA and P. aeruginosa, We Recommend These Empiric Antimicrobial Regimens
3.2.2.1. Without Severe Beta-Lactam Allergy (Strong Recommendation, High Quality of Evidence)
- Preferred regimens: Anti-pneumococcal beta-lactam (ceftriaxone 1 - 2 g daily and cefotaxime 1 - 2 g every 8 hours) + macrolide (500 mg azithromycin on the first day and 250 mg per day (preferred) or 500 mg clarithromycin twice daily). Fluoroquinolones can be our alternative choice of macrolides for those who have contraindications to both macrolides due to high antibiotic resistance rate to tetracyclines.
- Alternative: Monotherapy with a respiratory FQ; according to our data: levofloxacin 500 or 750 mg daily due to a lack of data about moxifloxacin 400 mg daily.
3.2.2.2. With a History of Severe Beta-Lactam Allergy
We recommend monotherapy with respiratory FQs; according to our data: levofloxacin 500 or 750 mg daily due to a lack of data about moxifloxacin 400 mg daily (strong recommendation, high quality of evidence).
3.3. In Patients with Risk Factors for MRSA
We recommend the following empiric treatment regimens:
3.3.1. With or Without Severe Beta-Lactam Allergy (Strong Recommendation, Moderate Quality of Evidence)
Add anti-MRSA, vancomycin (15 mg/kg twice daily, adjust according to the trough levels), or linezolid (oral or IV, 600 mg every 12 h) to the above regimens. Vancomycin is preferred due to available data about resistance patterns, and trough concentrations should be drawn within 60 minutes before administering the fourth dose for therapeutic monitoring.
3.4. In Patients with CAP and with Risk Factors for P. Aeruginosa, We Recommend These Empiric Antimicrobial Regimens
3.4.1. Without Severe Beta-Lactam Allergy (Weak Recommendation, Low Quality of Evidence)
Antipseudomonal/anti-pneumococcal beta-lactam (imipenem 500 mg every 6 hours, meropenem 1g every 8 hours) + anti-pseudomonococcal FQ (ciprofloxacin 400 mg every 8 hours, levofloxacin 500 or 750 mg daily).
3.4.2. With Severe Beta-Lactam Allergy €: (Strong Recommendation, Low Quality of Evidence)
Levofloxacin 500 or 750 mg daily + aminoglycoside (gentamicin)
3.5. Among Patients with CAP and with Risk Factors for MRSA and P. Aeruginosa
We recommend these empiric treatment regimens:
3.5.1. Without Severe Beta-Lactam Allergy € (Strong Recommendation, Low Quality of Evidence)
- Anti-MRSA◊: Vancomycin (15 mg/kg every 12 h, adjust based on levels) or linezolid (oral or IV, 600 mg every 12 h); vancomycin is preferred due to available data about resistance patterns, and trough concentrations should be drawn within 60 minutes before the administration of the fourth dose for therapeutic monitoring + antipseudomonal/anti-pneumococcal beta-lactam (imipenem 500 mg q6 hours, meropenem 1g q8 hours) + anti-pseudomonococcal FQ (ciprofloxacin 400 mg every 8 hours, levofloxacin 500 or 750 mg daily).
3.5.2. With Severe Beta-Lactam Allergy € (Strong Recommendation, low Quality of Evidence)
- Anti-MRSA◊: Vancomycin (15 mg/kg twice daily, adjust according to the trough levels) or linezolid (oral or IV, 600 mg every 12 h) (vancomycin is preferred due to available data about resistance patterns, and trough concentrations should be drawn within 60 minutes before the administration of the fourth dose for therapeutic monitoring) + levofloxacin 500 or 750 mg daily + aminoglycoside (gentamicin).
3.5.3. Summary of the Evidence
Anti-pneumococcal beta-lactams are available in Iran, including ceftriaxone, cefotaxime, and ampicillin-sulbactam. As a result of published studies in Iran, for S. pneumonia, cefotaxime with 0% - 42.5% and ceftriaxone with 0% - 31.5%, antimicrobial resistance, were recognized as antibiotics with minimum antibiotic resistance rate, respectively (14).
For M. catarrhalis, studies reported resistance rates against ceftriaxone as 6.2% and ampicillin as 84.4%, 70%, 0%, and 100% (13-15) and for H. influenza, ampicillin had a resistance rate of 43.43% and 66.6%, ceftriaxone 11.1%, 28.6%, and 0%, and cefotaxime 11.1% (12, 20, 21). In conclusion, the lowest resistance levels were against ceftriaxone, followed by cefotaxime and ampicillin for patients with CAP.
Pseudomonas is not a pathogen in CAP and is never considered a primary etiology. Only among a small proportion of patients with significant risk factors (Figure 2) can clinicians "consider" anti-pseudomonal regimens in addition to the main therapeutics. Among anti-pseudomonal/anti-pneumococcal beta-lactams, piperacillin-tazobactam, cefepime, ceftazidime, imipenem, and meropenem are available in Iran. But according to accomplished studies, the resistance rate seems high in all anti-pseudomonal regimens, particularly in cephalosporins (ceftazidime and cefepime) (28). The results of studies are heterogeneous, and data regarding pure community-acquired Pseudomonas are unavailable. However, data reveal three main mechanisms of beta-lactam resistance: Extended-spectrum beta-lactamase (ESBL), Metallo β-lactamases (MBL), and plasmidic AmpC β-lactamases among Pseudomonas isolates in Iran. Among monobactams, we omitted aztreonam from CAP treatment due to data about the high antibiotic resistance rate and inaccessibility in Iran (28).
Empiric antibiotic selection in inpatient setting: This figure is adjusted of community-acquired pneumonia UpToDate and IDSA algorithms for treatment of outpatients according our published data and resistance antibiotic rate in Iran. 1 Methicillin sensitive Staphylococcus aureus (MSSA) Risk factor: • Influenza active in community • Structural lung disease (eg, bronchiectasis) • Endobronchial obstruction • Injection drug use • Gram-positive cocci in clusters on good-quality sputum Gram stain. 2 MRSA risk factor (1): Strong risk factors that indicate need for empiric therapy: • Recognized colonization or previous infection with MRSA Other factors that raise suspicion for MRSA and may indicate need for empiric therapy depending on local prevalence and overall clinical assessment: • Recent hospitalization or antibiotic use, particularly hospitalization with receipt of IV antibiotics in the prior 3 months • Recent influenza-like illness • Necrotizing or cavity pneumonia • Presence of empyema • Risk factors for MRSA colonization: • End stage renal disease • Patients who are men who have sex with men • Injection drug use • Living in crowded conditions • Incarceration • Contact sport participation. 3 When patient has a contraindication to macrolide, the FQs are choice. 4 Individuals with a past reaction to penicillin that was mild (not Stevens Johnson syndrome, toxic epidermal necrolysis, or drug reaction with eosinophilia and systemic symptoms [DRESS]) and did not have features of an immunoglobulin (Ig) E-mediated reaction can receive a broad-spectrum third- or fourth-generation cephalosporins or carbapenems safely. 5Pseudomonas risk factor: Strong risk factors that indicate need for empiric therapy (1): • Hospitalization and treatment with parenteral antibiotics in the prior 3 months • Know colonization or prior infection with pseudomonas in patients with Structural lung disease (eg, bronchiectasis) Other factors that raise suspicion for Pseudomonas and may indicate need for empiric therapy depending on local prevalence and overall clinical assessment (29-33): • Structural lung abnormalities (eg, bronchiectasis, cystic fibrosis)• Immunosuppression • Frequent COPD exacerbations requiring frequent glucocorticoid or antibiotic use • Recent antibiotic use of any kind • Recent hospitalization or stay in a long-term care facility.
The antibiotic resistance rate against levofloxacin for respiratory FQ was estimated at 0% for both M. catarrhalis and H. influenza (12, 14, 21).
Antimicrobial resistance rates for anti-MRSA, including vancomycin and linezolid, have limited data in Iran. In this regard, Shokouhi et al. evaluated the antimicrobial susceptibility of CA-MRSA among Iranian patients and reported the prevalence of nasal carrier rate of S. aureus and CA-MRSA 22% and 1.25%, respectively. Based on the mentioned study, all 25 MRSA isolates from 440 S. aureus samples were susceptible to vancomycin and linezolid (100%), followed by SMX-TMP (68%), levofloxacin (48%), clindamycin, erythromycin (44%), and doxycycline (40%), (34). Oral linezolid can be replaced with a parenteral dosage form due to the bioavailability of 100% from the oral route of administration.
Based on several previous studies, the resistance rate of vancomycin against S. pneumonia was reported to be 0%, 1.5%, 7%, and 53%, and vancomycin was rated as the antibiotic with the least resistance rate (19).
The antibiotic resistance rates of ciprofloxacin and levofloxacin as anti-pseudomonococcal FQs were evaluated in different studies. Ciprofloxacin had a resistance rate of 0-11% for S. pneumonia, 0% and 70% for M. catarrhalis, and 0% and 57.1% for H. influenza. Likewise, levofloxacin was sensitive against M. catarrhalis and H. influenza with a 0% resistance rate (12-15, 19-21).
Gentamicin as an aminoglycoside has 0% and 70% against M. catarrhalis, and 0%, 22.8%, 46%, 63%, 76.7%, and 94% against S. pneumoniae in different studies (13, 15, 19).
4. Research Needed in Iran
There are limited head-to-head randomized clinical trials and high-quality evidence for treating patients with CAP. Furthermore, we need clinical trials comparing various antimicrobial regimens for outpatients and inpatients, assessing the incidence of antibiotics' adverse effects, publishing the results of antibiograms with broad-spectrum antibiotics, and estimating the prevalence of specific pathogens for detecting antimicrobial susceptibility.
![Empiric antibiotic selection in inpatient setting: This figure is adjusted of community-acquired pneumonia UpToDate and IDSA algorithms for treatment of outpatients according our published data and resistance antibiotic rate in Iran. <sup>1</sup> Methicillin sensitive <i>Staphylococcus aureus</i> (MSSA) Risk factor: • Influenza active in community • Structural lung disease (eg, bronchiectasis) • Endobronchial obstruction • Injection drug use • Gram-positive cocci in clusters on good-quality sputum Gram stain. <sup>2</sup> MRSA risk factor (<a href="#A133876REF1">1</a>): Strong risk factors that indicate need for empiric therapy: • Recognized colonization or previous infection with MRSA Other factors that raise suspicion for MRSA and may indicate need for empiric therapy depending on local prevalence and overall clinical assessment: • Recent hospitalization or antibiotic use, particularly hospitalization with receipt of IV antibiotics in the prior 3 months • Recent influenza-like illness • Necrotizing or cavity pneumonia • Presence of empyema • Risk factors for MRSA colonization: • End stage renal disease • Patients who are men who have sex with men • Injection drug use • Living in crowded conditions • Incarceration • Contact sport participation. <sup>3</sup> When patient has a contraindication to macrolide, the FQs are choice. <sup>4</sup> Individuals with a past reaction to penicillin that was mild (not Stevens Johnson syndrome, toxic epidermal necrolysis, or drug reaction with eosinophilia and systemic symptoms [DRESS]) and did not have features of an immunoglobulin (Ig) E-mediated reaction can receive a broad-spectrum third- or fourth-generation cephalosporins or carbapenems safely. <sup>5</sup><i>Pseudomonas</i> risk factor: Strong risk factors that indicate need for empiric therapy (<a href="#A133876REF1">1</a>): • Hospitalization and treatment with parenteral antibiotics in the prior 3 months • Know colonization or prior infection with pseudomonas in patients with Structural lung disease (eg, bronchiectasis) Other factors that raise suspicion for <i>Pseudomonas</i> and may indicate need for empiric therapy depending on local prevalence and overall clinical assessment (<a href="#A133876REF29">29</a>-<a href="#A133876REF33">33</a>): • Structural lung abnormalities (eg, bronchiectasis, cystic fibrosis)• Immunosuppression • Frequent COPD exacerbations requiring frequent glucocorticoid or antibiotic use • Recent antibiotic use of any kind • Recent hospitalization or stay in a long-term care facility. Empiric antibiotic selection in inpatient setting: This figure is adjusted of community-acquired pneumonia UpToDate and IDSA algorithms for treatment of outpatients according our published data and resistance antibiotic rate in Iran. <sup>1</sup> Methicillin sensitive <i>Staphylococcus aureus</i> (MSSA) Risk factor: • Influenza active in community • Structural lung disease (eg, bronchiectasis) • Endobronchial obstruction • Injection drug use • Gram-positive cocci in clusters on good-quality sputum Gram stain. <sup>2</sup> MRSA risk factor (<a href="#A133876REF1">1</a>): Strong risk factors that indicate need for empiric therapy: • Recognized colonization or previous infection with MRSA Other factors that raise suspicion for MRSA and may indicate need for empiric therapy depending on local prevalence and overall clinical assessment: • Recent hospitalization or antibiotic use, particularly hospitalization with receipt of IV antibiotics in the prior 3 months • Recent influenza-like illness • Necrotizing or cavity pneumonia • Presence of empyema • Risk factors for MRSA colonization: • End stage renal disease • Patients who are men who have sex with men • Injection drug use • Living in crowded conditions • Incarceration • Contact sport participation. <sup>3</sup> When patient has a contraindication to macrolide, the FQs are choice. <sup>4</sup> Individuals with a past reaction to penicillin that was mild (not Stevens Johnson syndrome, toxic epidermal necrolysis, or drug reaction with eosinophilia and systemic symptoms [DRESS]) and did not have features of an immunoglobulin (Ig) E-mediated reaction can receive a broad-spectrum third- or fourth-generation cephalosporins or carbapenems safely. <sup>5</sup><i>Pseudomonas</i> risk factor: Strong risk factors that indicate need for empiric therapy (<a href="#A133876REF1">1</a>): • Hospitalization and treatment with parenteral antibiotics in the prior 3 months • Know colonization or prior infection with pseudomonas in patients with Structural lung disease (eg, bronchiectasis) Other factors that raise suspicion for <i>Pseudomonas</i> and may indicate need for empiric therapy depending on local prevalence and overall clinical assessment (<a href="#A133876REF29">29</a>-<a href="#A133876REF33">33</a>): • Structural lung abnormalities (eg, bronchiectasis, cystic fibrosis)• Immunosuppression • Frequent COPD exacerbations requiring frequent glucocorticoid or antibiotic use • Recent antibiotic use of any kind • Recent hospitalization or stay in a long-term care facility.](https://services.brieflands.com/cdn/serve/3170b/4ebc35c4dab9c63f70fc230999462026e617f0ae/archcid-18-1-133876-i002-preview.webp)