1. Background
Urinary tract infection (UTI) is highly prevalent in developing countries, making it one of the most common illnesses among hospitalized patients, accounting for over 40% of cases (1).
Urinary tract infections, caused by different types of harmful microorganisms, are commonly found throughout the urinary system, including the kidney, ureter, bladder, and urethra. The presence of UTIs in these areas is widespread. The Enterobacteriaceae family of bacteria is the most commonly identified pathogen responsible for UTIs. This family includes Escherichia coli, which accounts for the majority of UTI cases (reaching 77%), particularly among young women. Other bacteria such as Staphylococcus aureus, Proteus species, Acinetobacter spp, Klebsiellapneumoniae, Pseudomonas aeruginosa, and Enterococcus faecalis also contribute to the bacterial landscape of UTIs (2, 3). These bacteria possess virulence factors that contribute to their pathogenicity as infectious agents (4).
Although E. faecalis is typically a gram-positive bacterium found in the gut as a commensal, it is commonly associated with various significant human infections. These infections include UTIs, endocarditis, bacteremia, and wound infections. Urinary tract infections caused by E. faecalis are highly prevalent, accounting for approximately 110 000 cases annually. Many of these infections are acquired in health care settings (nosocomial). Enterococcus faecalis has been identified as the third-most significant uropathogen in terms of causing intermittent and chronic UTIs among patients in intensive care units (ICUs) (5).
In recent years, there has been a renewed focus on exploring plants as potential sources of antimicrobial agents. This interest stems from their traditional medicinal use and the reliance of a significant portion of the global population, particularly in developing countries, on plants for the treatment of both infectious and non-infectious diseases. The World Health Organization (WHO) estimates that over 80% of people worldwide utilize various plant extracts in folk medicine (6). One of the key advantages of plant extracts is their perceived safety and lack of side effects. As a result, numerous studies have investigated the antimicrobial activity of plant extracts, aiming to identify alternative drugs to combat resistant organisms (7). Syzygium aromaticum, commonly known as clove, is an aromatic plant belonging to the family Myrtaceae. It is typically found in the form of dried flower buds. Clove has been widely recognized for its various therapeutic applications, including its ability to protect against internal parasites, act as an antiseptic, and exhibit antimicrobial properties against both fungi and bacteria. It is thought that clove's antimicrobial properties come from its ability to damage the cell wall and membrane, which breaks down cells, frees their contents, and stops the proton motive force (8).
Furthermore, cloves possess various additional beneficial properties, such as anti-mutagenic, anti-inflammatory, antioxidant, antiulcerogenic, and antithrombotic effects. It has also been reported that clove exhibits bactericidal activity without promoting the development of bacterial resistance (9).
2. Objectives
The current study looked at how well the ethanolic extract from the S. aromaticum plant killed clinical isolates of E. faecalis, a type of bacteria that causes UTIs.
3. Methods
3.1. Subjects and Sample Collection
The present study was conducted in the Microbiology Department, Al-Maarif University College, Anbar, Iraq. A total of 251 midstream urine specimens were collected from women aged 17 to 52 years who were diagnosed with UTIs based on symptoms. The specimens were collected using sterile plastic containers following the standard clean-catch midstream procedure. The participants were attending hospitals in Baghdad, Iraq, specifically Baghdad Teaching Hospital, Ghazi Hariri Hospital, Central Laboratories in Medical City, and Al-Yarmouk Hospital, during the period from September 2021 to January 2022. To maintain sample integrity, each specimen was promptly transported to the laboratory under appropriate cooling conditions for further analysis.
3.2. Isolation and Identification of Enterococcus faecalis
The urine samples were initially subjected to centrifugation at 1500 rpm for 5 min. Following centrifugation, the supernatant was carefully removed, and the resulting pellet was cultured in a Brain Heart Infusion (BHI) broth medium. The cultures were then incubated at 37ºC for 24 h to facilitate bacterial growth. Subsequently, the cultured samples were streaked onto general and differential culture media, including blood agar and Pfizer Selective Enterococcus (PSE) agar, which serves as the selective primary medium for E. faecalis. The PSE agar changes color to black in the presence of E. faecalis. The streaked plates were then incubated at 37ºC for 24 h to allow for bacterial growth and observation. To further examine the colonies obtained, they were re-streaked on m-EI Chromogenic Agar Base Modified medium (Candalab) to identify the presence of greenish-blue colonies. These plates were then incubated for an additional 24 h at 37ºC (10). The confirmation of E. faecalis growth was based on various criteria, including morphological characteristics such as colony shape, size, margin, consistency, texture, edges, height, and color. Microscopic features, biochemical tests, and the Vitek II system were also employed in the diagnosis of E. faecalis.
3.3. Antibacterial Screening for the Effectiveness of Clove Plant Extract
The preparation of the plant extract followed the methods described in references (11) and (12). The antimicrobial activity of the plant extract was assessed using the agar well diffusion test following the procedures outlined in references (13) and (14). This method was selected to evaluate the antimicrobial efficacy of the plant extract in the present study. To summarize, the clove plant extract powder was dissolved in a suitable solvent, specifically diluted dimethyl sulfoxide (10% DMSO), to create a stock solution in a tube. The final concentration of the stock solution was 200 mg/mL. The solution was then filtered using a 0.22-millipore filter to remove any particulate matter or impurities. Serial dilutions were performed on the stock solution to obtain a range of concentrations. Starting with a stock solution that was 100 mg/mL, 100 mL of it was moved to another tube that already had 100 mL of 10% DMSO in it. This made the concentration 50% lower, bringing it back up to 100 mg/mL. The contents of the second tube were thoroughly mixed, and then 100 mL of aliquots from the second tube was transferred to a third tube, which also contained 100 mL of aliquots of 10% DMSO. This resulted in an additional 50% dilution of the antimicrobial, resulting in a concentration of 50 mg/mL. This process of dilution was continued in subsequent tubes to obtain concentrations of 25, 12.5, 6.25, and 3.125 mg/mL, using DMSO as the diluent. The described procedure was repeated to obtain other dilutions. The negative control in this experiment consisted of using the solvent DMSO. To conduct the test, young and pure culture of a previously identified bacterial isolate was utilized. The inoculum was prepared by transferring 3 - 5 well-isolated colonies grown on agar plates to sterile tubes containing 3 mL of normal saline. After that, the tubes were kept at 37°C for 2 h so that turbid growth could happen, which was equal to the standard turbidity of the McFarland standard tube number 0.5. This means that there were 1.5 × 108 colony-forming units (CFU)/mL. The bacterial suspension from the inoculum was collected using a sterile, disposable swab. The swab was then streaked onto Petri plates containing Muller-Hinton agar. The agar medium was prepared in accordance with the manufacturer's instructions and spread evenly across the surface of the plate to a thickness of approximately 4 mm. The swab was rotated 3 times at a 60° angle after each application, ensuring comprehensive coverage of the agar surface. Finally, the swab was moved uniformly along the edge of the agar surface. The plates were then allowed to air dry at room temperature. Following the preparation of the agar plates, a sterile cork borer was used to aseptically punch a well with a diameter of 6 mm in the agar surface. Approximately 20 μL of the plant extract solution was then transferred into the well of each Petri dish. The plates were subsequently incubated at 37°C for a period of 18 to 24 h. After incubation, the inhibition zones formed around the wells were measured using a digital vernier caliper, and the diameters of the zones of inhibition were recorded in millimeters.
4. Results and Discussions
4.1. Prevalence of Enterococcus faecalis Associated with Urinary Tract Infections
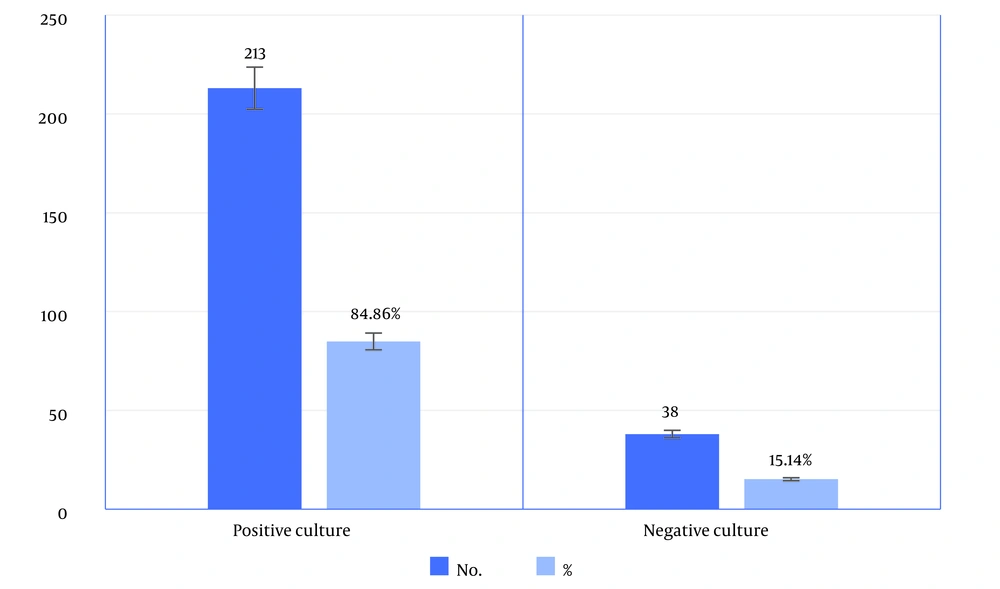
A total of 251 clinical urine samples were subjected to culture on various agar plates, including nutrient agar, MacConkey agar, blood agar, PSE agar, and m-EI Chromogenic Agar Base Modified agar. The purpose was to isolate and identify E. faecalis. Among the samples, 84.86% (213/251) displayed positive bacterial cultures, indicating the presence of E. faecalis. Conversely, no growth was observed in the remaining 15.14% (38/251) samples (Figure 1).
In our study, among 213 positive culture samples, only 16.43% (35/213) isolates belonged to E. faecalis, and 83.57% (178/213) isolates belonged to other genera of bacteria (Table 1); these results are consistent with the study conducted by Goel et al (15).
| Bacterial Isolates | No. (%) |
|---|---|
| Enterococcus faecalis | 16.43 (35) |
| Others | 83.57 (178) |
| Total | 100 (213) |
These results are consistent with previous findings reported by Ghaly et al (2009) (16) in Egypt. They found that only 9% of urine samples were contaminated with Enterococcus spp. Similarly, the results of Al-jmor's study (2012) conducted in Iraq (17) showed that E. faecalis accounted for 20.6% of the isolates obtained from urine samples. However, our findings were inconsistent with the study conducted in Pakistan by Hussain et al (2016) (18); they found that the most common pathogenic-isolated bacterium associated with UTIs was S. faecalis (70%). Similarly, a study conducted by Alebouyeh et al. (19) in Iran showed that E. faecalis constituted a significant proportion of the isolates obtained from urine samples, accounting for 75% of the total. This discrepancy in percentages may be attributed to several factors. First, the variation in percentages could be due to the difference in study populations. The study mentioned above included both males and females, whereas our study focused exclusively on women. Second, geographical distribution can play a role in the prevalence of bacterial species. The studies conducted in the Arab world, specifically in Iraq and Egypt, reported results that are similar to ours.
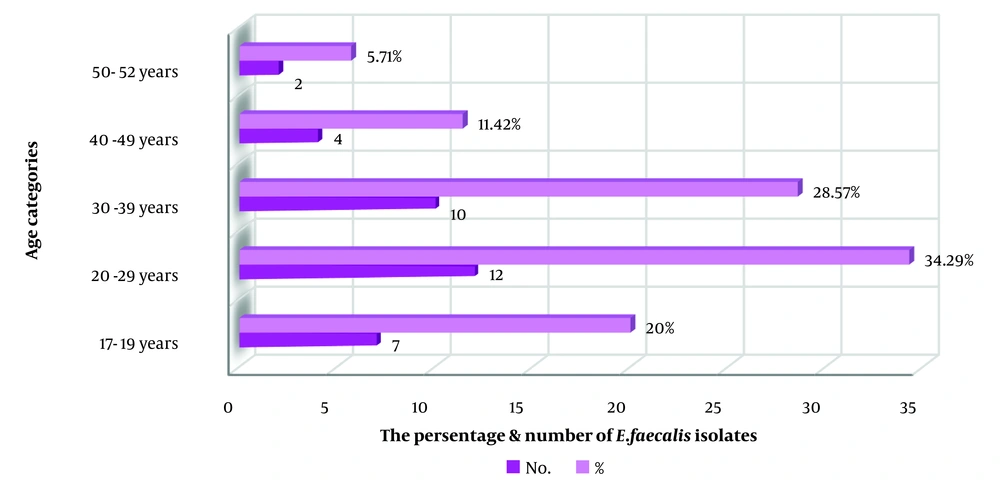
Based on Figure 2, it is evident that the highest percentage of E. faecalis isolates was observed among young women, particularly in the age group of 20-29 years, with a percentage of 34.29%. This was followed by the age group of 30 - 39 years, which recorded 28.57% of E. faecalis isolates compared to other age groups in the study. Our results were in good agreement with those of Hussain et al (2016) (18), showing that the highest percentage of E. faecalis isolates appeared among infertile individuals in the age groups of 15 - 24and 25 - 34 years, accounting for 9.16% and 17.5%, respectively; UTIs is often linked to the sexual activity increases at this age (11, 16).
4.2. Phenotypic Characterization and Microscopic Identification
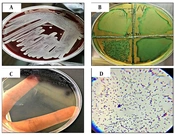
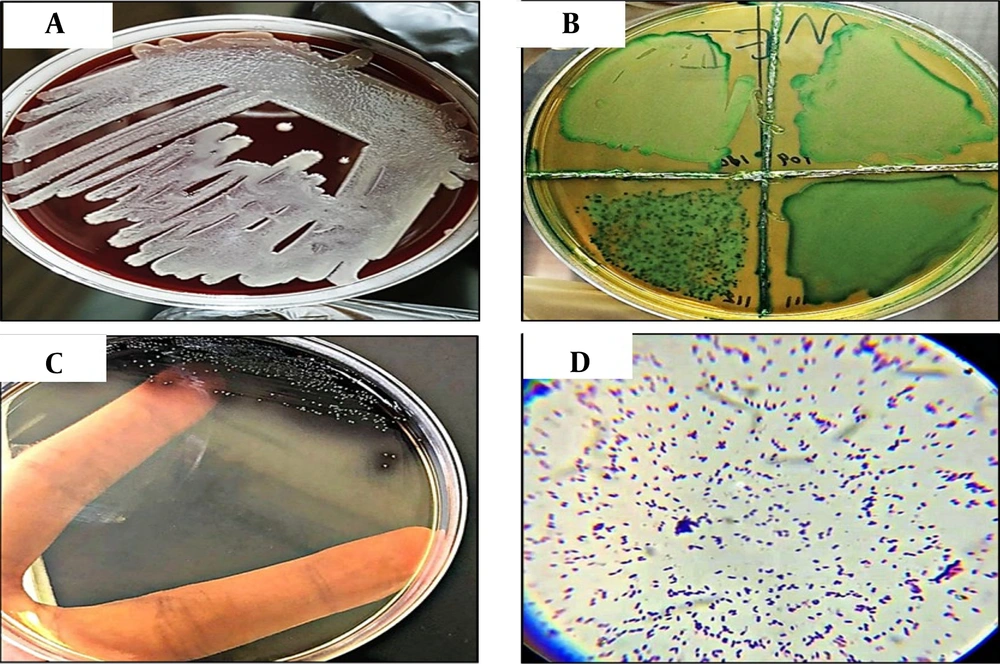
Enterococcus faecalis was phenotypically characterized and microscopically identified using bacteriological methods. This included assessing the colonial morphology (shape and color) and microscopic examination, particularly through Gram staining. The Gram stain allowed for the observation of bacterial cell morphology, cell arrangement, and the reaction to the stain (Figure 3).
The different aspects of Enterococcus faecalis identification. In panel (A), colonies of E. faecalis (gamma hemolytic) grown on blood agar at 37°C for 24 h are shown. Panel (B) displays E. faecalis colonies grown on Pfizer Selective Enterococcus (PSE) agar at 37°C for 24 h. In panel (C), the morphological appearance of E. faecalis colonies on PSE agar at 37°C for 24 h is depicted, showing a distinctive greenish-blue color. Finally, panel (D) presents the microscopic examination of E. faecalis after performing the Gram staining process. The microscopic image reveals cocci-shaped gram-positive (blue color) cells that are spherical or ovoid in shape. They are observed arranged singly, in pairs, or in short chains, and they do not possess spores.
4.3. Biochemical Identification
Biochemical tests were carried out according to (20), and the results of the biochemical tests are summarized in Table 2. These results are consistent with the results of Kadhem and Flayyih (2014) (21) and Hasson and Kadhem (2015) (20).
| Bacteria | Biochemical Tests | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalase | Oxidase | Indole | MR | VP | Citrate | BET | Urease | Growth at 10 and 40 ºC | Growth at 9.6 pH | Growth at 6.5% NaCl | |
| E. faecalis | - | - | - | - | + | - | + | - | + | + | + |
Abbreviations: MR, methyl red test; VP, Voges-Proskauer test; BET, bile esculin test; (-), negative result; (+), positive result.
4.4. Detection of Antimicrobial Susceptibility by Vitek II
The susceptibility testing of the bacterial isolates was performed using the Vitek II method, which involved evaluating their response to 14 specific antibiotics. Table 3 presents the resistance findings of the E. faecalis isolates during the current analysis against these antibiotics.
| Antibiotics | Resistant, No. % | Sensitive, No. % |
|---|---|---|
| Benzylpenicillin | 35 (100) | 0 (0) |
| Oxacillin | 29 (82.86) | 6 (17.14) |
| Gentamicin | 9 (25.71) | 26 (74.29) |
| Ciprofloxacin | 6 (17.14) | 29 (82.86) |
| Moxifloxacin | 2 (5.71) | 33 (94.29) |
| Erythromycin | 30 (85.71) | 5 (14.29) |
| Clindamycin | 35 (100) | 0 (0) |
| Teicoplanin | 13 (37.14) | 22 (62.86) |
| Vancomycin | 25 (71.43) | 10 (28.57) |
| Tetracycline | 12 (34.29) | 23 (65.71) |
| Tigecycline | 17 (48.57) | 18 (51.43) |
| Fusidic acid | 28 (80) | 7 (20) |
| Rifampicin | 27 (77.14) | 8 (22.86) |
| Trimethoprim/sulfamethoxazole | 11 (31.43) | 24 (68.57) |
Antibiotic therapy aims to minimize or remove pathogenic bacteria in the ejaculate, as well as to enhance irregular sperm parameters. Due to both intrinsic and acquired antibiotic resistance of bacterial agents that cause infections of the urinary tract in patients, antimicrobial therapy should be guided by sensitivity test results (22).
According to the data presented in Table 3, it is evident that moxifloxacin, ciprofloxacin, and gentamicin exhibited the highest effectiveness as antimicrobial agents against the bacterial isolates. Out of the total number of isolates (35), 33 isolates were sensitive to moxifloxacin, 29 isolates were sensitive to ciprofloxacin, and 26 isolates were sensitive to gentamicin. Trimethoprim/sulfamethoxazole, tetracycline, and teicoplanin were also very effective. However, benzylpenicillin and clindamycin were the least effective, as resistance to this antibiotic appeared in 35 (100%) isolates. Other antibiotics were successful at various levels. The results of the current study did not agree with Hussain et al. (2016) (18), who found that the percentage of resistance of E. faecalis isolates to ciprofloxacin and gentamicin was 85.9% and 90.09%, respectively, while the resistance of E. faecalis to these antibiotics was low in our study (17.14% and 25.71%, respectively). Also, the resistance percentage to vancomycin was high (71.43%) compared to the results of (8), where the resistance percentage of isolates was very low (1.86%). The studies reported by (21, 23) demonstrated similar findings regarding vancomycin resistance. They observed percentages of vancomycin-resistant strains ranging from 50% to 90.06%. These results closely align with our own findings, indicating a comparable prevalence of vancomycin resistance.
One significant factor contributing to the persistence of Enterococcus in hospital settings is its inherent resistance to numerous commonly prescribed antibiotics. Moreover, of greater concern is its capability to develop resistance to all existing antibiotics, either through genetic mutation or the transfer of plasmids and transposons (24).
4.5. Screening the Antibacterial Activity of Clove Plant Extract
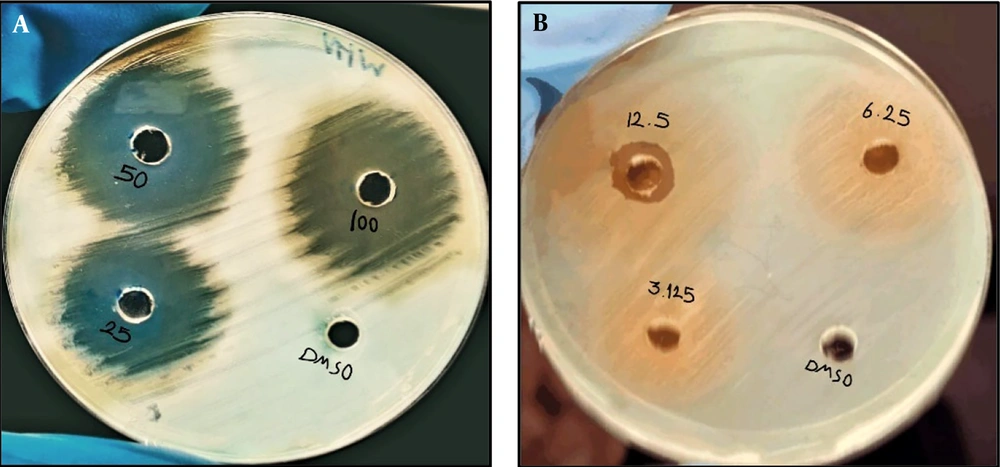
The agar well diffusion method was employed to assess the antibacterial activity of the ethanolic extract derived from the clove plant against E. faecalis isolates. The results of this investigation are summarized in Table 4 and depicted in Figure 4.
| Concentrations of Clove Extract (mg/mL) | Isolates; No. (%) | Inhibition Zone Range (mm) | |
|---|---|---|---|
| Inhibition Zone | No Inhibition Zone | ||
| 100 | 35 (100) | 0 (0) | 21.13 - 19.65 |
| 50 | 35 (100) | 0 (0) | 17.89 - 14.27 |
| 25 | 35 (100) | 0 (0) | 13.36 - 12.52 |
| 12.5 | 35 (100) | 0 (0) | 8.26 - 7.14 |
| 6.25 | 0 (0) | 35 (100) | - |
| 3.125 | 0 (0) | 35 (100) | - |
The impact of various concentrations of clove plant extract on Enterococcus faecalis isolates. The right photo displays the effects of concentrations of 100, 50, and 25 mg/mL, while the left photo shows the effects of concentrations of 12.5, 6.25, and 3.125 mg/mL. The bacterial growth on Moller Hinton agar after 24 h of incubation at 37°C is depicted. The control group was treated with the solvent dimethyl sulfoxide.
From Table 4, it is evident that the effect of the ethanolic extract of S. aromaticum varies according to the concentrations tested. The highest effectiveness was observed at a concentration of 100 mg/mL against the bacteria isolated during the current study. This concentration resulted in an inhibition zone diameter ranging from 21.13 to 19.65 mm, followed by concentrations of 50, 25, and 12.5 mg/mL, respectively. However, the concentrations of 6.25 and 3.125 mg/mL had no anti-bacterial activity, and no inhibition zone was revealed. Moreover, the results showed that the sensitivity of bacteria isolates to the clove extract was greater or similar to their sensitivity to some antibiotics. This can be explained by 2 possible mechanisms. First, it is possible that the bacteria tested were not previously exposed to these extracts, making them more susceptible to their antimicrobial properties. In this case, the bacteria may not have developed resistance mechanisms against the specific compounds present in the extract. Second, S. aromaticum may have a chemical affinity for interacting with specific components of the bacterial cells. There could be receptors or transporters on the bacterial cell wall that facilitate the entry of the extract into the cell. Once inside, the extract may interfere with the action of active enzymes or other biological molecules, leading to the inhibition of bacterial growth (25).
5. Conclusions
The ethanolic extract derived from the aromatic dried flower buds of clove (S. aromaticum) showed great potential as an effective antibacterial agent. Additionally, this study suggests that the use of this plant could be a promising therapeutic option for addressing UTIs, especially in the context of increasing concerns about drug resistance.