1. Introduction
Leuconostoc species are gram-positive cocci naturally found in dairy products and vegetables and are rarely present in human excreta and tissues (1). General resistance to glycopeptides, such as teicoplanin and vancomycin, is a characteristic feature of Leuconostoc species. This antimicrobial resistance is due to the absence of these agents in the bacterial cell wall (2). The resistance mechanism appears to be chromosomally mediated and involves the production of peptidoglycan precursors ending in D-alanine-D-lactate, making these bacteria naturally resistant to glycopeptides. Leuconostoc species carry a gene that changes the D-ala-D-ala amino acid chain to D-ala-D-lactate, facilitating peptidoglycan wall formation and severely limiting vancomycin binding (3, 4). A variety of infections have been reported among immunocompromised patients, though the first case of Leuconostoc infection was reported in 1985 (1, 5). In patients with respiratory tract infections, it has been rarely reported. Here, we present a case report of Leuconostoc pseudomesenteroides as a cause of lower respiratory tract infection in a patient with tracheostomy.
2. Case Presentation
Prior to reporting this case, consent was obtained from the patient. A 69-year-old male patient with a known history of type 2 diabetes mellitus and hypertension was admitted to the hospital following a road traffic accident, during which he sustained a head injury associated with bilateral ear bleeding. Clinical and radiological evaluation diagnosed the patient with diffuse axonal injury and bilateral subdural hematoma. A routine abdominal/pelvic ultrasound detected no obvious sonological abnormalities.
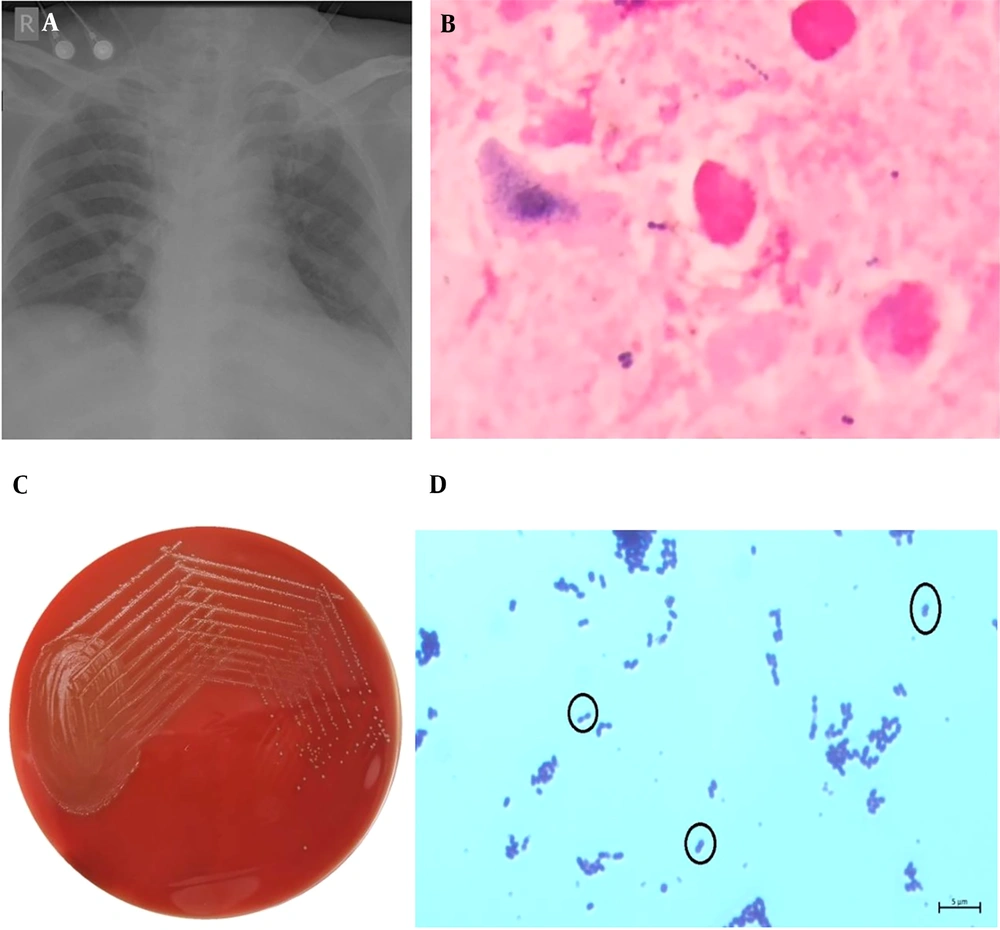
The patient underwent a tracheostomy due to dropping blood oxygen saturation levels (SpO2) and was managed conservatively during the course of treatment. Regular physiotherapy was provided, tracheostomy care was maintained, and SpO2 saturation was sustained at room air. On the 9th day of admission, the patient developed a fever of 37.9°C, his white blood cell count rose to 13,100/mm³ (N: 88%), and C-reactive protein levels elevated to 14.68 mg/dL. A chest X-ray exhibited bilateral lung infiltrates (Figure 1A). Suspected of having acquired an infection, peripheral blood samples for blood culture and an endotracheal aspirate sample were taken for further microbiological evaluation.
A, chest X-ray (Anteroposterior view) of the patient on 9st day of admission exhibiting bilateral lung infiltrates. B, endotracheal aspirate sample on gram-staining demonstrates presence of gram-positive cocci in pairs and leukocytes, as observed under oil immersion (100X); C, white, small and raised alpha-haemolytic colonies isolated in 5% sheep blood agar; D, gram-stain of L. pseudomesenterides colonies from blood agar show gram-positive cocci in pairs as observed under oil immersion (100X).
2.1. Microbiological Identification
Blood samples were incorporated into BACT/ALERT culture medium and incubated under normal environmental conditions at 35°C in an automatic BACT/ALERT® 3D culture machine (BioMérieux, Marcy L’Etoile, France). After 5 days of incubation, the blood cultures showed no growth of microorganisms.
Endotracheal aspirate samples were inoculated onto 5% sheep blood agar as well as MacConkey agar plates and incubated at 37⁰C for 18 - 24 hours. Numerous gram-positive cocci in pairs (Figure 1B), numerous leukocytes (>25 neutrophils/10X), and few epithelial cells (<10 squamous epithelial cells/10X) were seen in the Gram stain of the endotracheal aspirate, signifying adequate sample collection. High growth of white, small, smooth, round (0.5 mm - 1 mm), and raised alpha-hemolytic colonies was isolated on 5% sheep blood agar using the streak plate isolation method (Figure 1C), showing >105 CFU/ml on the semi-quantitative culture method, with no growth on MacConkey agar. The catalase test was negative. The bacteria were identified as Leuconostoc pseudomesenteroides with 99% probability by the VITEK 2 Compact System (BioMérieux, Marcy L’Etoile, France) using the gram-positive identification card. Antimicrobial susceptibility tests were conducted according to Clinical and Laboratory Standards Institute standards, with media and antibiotic breakpoints per protocol recommendations (6). Routine testing for susceptibility was not done, as Leuconostoc is intrinsically resistant to vancomycin (5). The mechanism of resistance seems to be chromosomally mediated and contrasts with the mechanism demonstrated by vancomycin-resistant enterococci. Because of its pentapeptide cell wall precursors ending in alanine-lactate rather than in the alanine-alanine dipeptide, which is the binding site for vancomycin in susceptible gram-positive cocci, Leuconostoc is intrinsically resistant to vancomycin (3, 4). The microorganism was susceptible to ampicillin (≤8 µg/mL), penicillin (≤8 µg/mL), and chloramphenicol (≤8 µg/mL).
After bacterial identification and antibiotic susceptibility testing were verified, ampicillin was administered intravenously, appropriate for the susceptibility of L. pseudomesenteroides. On the 6th day of antibiotic administration, the patient's white blood cell count and C-reactive protein values started to recede toward normal ranges. As his clinical symptoms began to improve, a repeat endotracheal aspirate culture test was conducted on the 10th day of antibiotic administration, and no bacteria were identified. He was eventually discharged from the hospital following post-traumatic rehabilitation in normal clinical condition.
3. Discussion
Leuconostoc comprises 29 other species. Leuconostoc species grow in pairs and chains and are gram-positive, facultative anaerobic coccus or coccobacillus, catalase and oxidase negative (Figure 1D). In routine biochemical testing in microbiology laboratories, it forms colonies that are often morphologically misidentified as Enterococcus or Streptococcus viridans. Since misidentification of Leuconostoc species as Streptococcus, Enterococcus, or Lactobacillus is commonly seen, it is important to isolate and distinguish between these species (7). Previously, Leuconostoc species were given limited clinical importance and considered non-pathogenic (8). However, with the increase in the number of infections attributed to Leuconostoc spp., they are now considered to be opportunistic pathogens, correlating with bacteremia in severely immunocompromised hosts (9, 10).
Though the specific mechanism of the disease is not well-investigated, risk factors for infection due to Leuconostoc include parenteral nutrition, surgical procedures, hepatic failure, central venous catheters, hemodialysis, and previous antibiotic therapy. The digestive tract, respiratory tract, and skin are believed to play important roles as ports of entry (4). In our case study, a major differential diagnosis involved aspiration pneumonia, given the history of a road traffic accident leading to significant head injury, resulting in lung parenchymal consolidation in a type II diabetic patient with reduced immunity. The approach involves appropriate diagnostic and prompt therapeutic measures, keeping in view the unusual bacterial pathogens that can cause lower respiratory tract infections.
Selecting antimicrobial agents to treat Leuconostoc infections is challenging as there are no established standards. Leuconostoc is intrinsically resistant to vancomycin (6). Clindamycin, linezolid, macrolides, aminoglycosides, cephalosporins, and tetracyclines have also been used to treat Leuconostoc infections, but the treatment of choice seems to be penicillin or ampicillin (4).
Unlike other reported clinical cases, excluding routine infusion with indwelling needles, the patients in our study did not use parenteral nutrition or deep vein catheterization. In peripheral blood culture, no pathogenic microorganisms were detected. It is not consistently associated with bloodborne infections as previously reported. However, the patient was on endotracheal intubation, providing a route for direct access to the respiratory system (6).
Leuconostoc species should be considered potential pathogens, particularly in immunocompromised hosts. Their frequent misidentification as other Streptococcaceae suggests that the clinical spectrum of infections caused by these organisms may be much broader than currently understood. Therefore, all gram-positive bacteria isolated from sterile body locations should undergo susceptibility testing.
Leuconostoc is not typically seen in regular human microbiota. However, the presence of this organism has recently been observed in immunocompromised patients and those with associated comorbidities. The prevalence of respiratory tract infections, coupled with a large population already infected with tuberculosis, makes managing this condition difficult. Moreover, diagnosing this condition is challenging due to the heterogeneity of the bacteria in systemically compromised patients. Empirical treatment modalities can be improved by employing biopsies, which can assist clinicians in diagnosing and understanding the pathological and microbiological mode of spread.