1. Introduction
Chronic migraine (CM), with a global prevalence of 1.4 - 2.2%, is a debilitating neurological condition with few treatment options (1). It is characterized by headaches occurring at least 15 days per month for at least three consecutive months, with at least eight days a month meeting diagnostic criteria for migraines (2). Since receiving approval from the Food and Drug Administration in 2011, botulinum toxin has been commonly used to manage CM. Considering the global prevalence of migraines, the increasing use of botulinum toxin for preventive treatment, and the global distribution of COVID-19 (Coronavirus Disease 2019) vaccinations, it has become crucial to understand the potential impact of these vaccines on the efficacy of botulinum toxin in managing migraines.
Since the outbreak of COVID-19 three years ago and the subsequent development of various vaccines, some adverse effects of these vaccinations are yet to be fully understood. Among 1.57 million clinical trial participants, headaches after COVID-19 vaccination emerged as the third most frequent adverse event (3). Moreover, the incidence of post-vaccination headaches was generally higher in individuals with pre-existing headache conditions (4).
This case series examines five CM patients who initially responded favorably to abobotulinum toxin type A (BTX-A) treatment. However, they experienced an increase in headaches days after receiving COVID-19 vaccines. They indicated a potential decrease in the therapeutic efficacy of botulinum toxin, due to an unexpected surge in headache frequency, following COVID-19 vaccination.
2. Case Presentation
This study presents five cases from an ongoing randomized clinical trial conducted at the headache clinic of Sina Hospital, located in Tehran, Iran (ethics code: IR.TUMS.MEDICINE.REC.1400.1227, clinical trial registration number: IRCT20201104049265N3). Details of the clinical trial protocol, such as inclusion and exclusion criteria, can be accessed through the IRCT database. The clinical trial aims to assess the management of CM by botulinum toxin type A (BTX-A), evaluating the non-inferiority of Dyston (manufactured by Imen Vaccine Alborz) compared to Dysport (manufactured by IPSEN). The trial follows a fixed-site-fixed-dose injection protocol, similar to the Phase III Research Evaluating Migraine Prophylaxis Therapy (PREEMPT) clinical program (5). The target sample size of the clinical trial was set at 92 participants, to be allocated to two BTX-A groups in a 1:1 ratio through a simple random sampling method. The cases discussed here were chosen based on an observable decline in headache days after BTX-A treatment, followed by a noticeable relapse after receiving COVID-19 vaccinations.
Each participant had undergone a routine neurological examination and had shown no abnormalities in their magnetic resonance imaging (MRI). They were instructed to complete headache diaries from one month before toxin injection until the end of the clinical trial, which would be evaluated monthly to track the number of headache days, intensity of headaches, and use of abortive medications. The trial specified that there would be no changes in non-prohibited concurrent pharmacologic and non-pharmacologic treatments throughout the study.
All patients were diagnosed with CM according to the third edition of the International Classification of Headache Disorders (ICHD-3) criteria (6). They were enrolled in the trial after meeting the eligibility criteria and providing their informed consent. Each participant received a 500 IU BTX-A injection administered by the same physician using a uniform protocol. The patients were followed up with face-to-face visits every four weeks for three months post-BTX-A injection. The demographic and baseline data of the patients are presented in Table 1.
| Case Number | Age | Sex | Weight (kg) | BMI (Kg/m2) | Smoking | Family History of Migraine | Diagnosis | Overused Medication(s) | Age of Migraine Diagnosis | Age of Chronic Migraine Onset |
|---|---|---|---|---|---|---|---|---|---|---|
| Case one | 50 | Female | 60 | 23.43 | No | Yes | CM+MOH (without aura) | Acetaminophen, NSAIDs, ergot alkaloid, caffeine | 40 | 50 |
| Case two | 31 | Female | 70 | 29.13 | No | No | CM (without aura) | None | 27 | 30 |
| Case three | 41 | Female | 63 | 23.14 | No | Yes | CM+MOH (without aura) | Triptan, NSAIDs | 31 | 39 |
| Case four | 40 | Female | 62 | 24.83 | No | Yes | CM+MOH (without aura) | Triptan, NSAIDs | 18 | 40 |
| Case five | 45 | Female | 64 | 24.30 | No | Yes | CM (without aura) | - | 25 | 30 |
Baseline and Demographic Data of the Cases
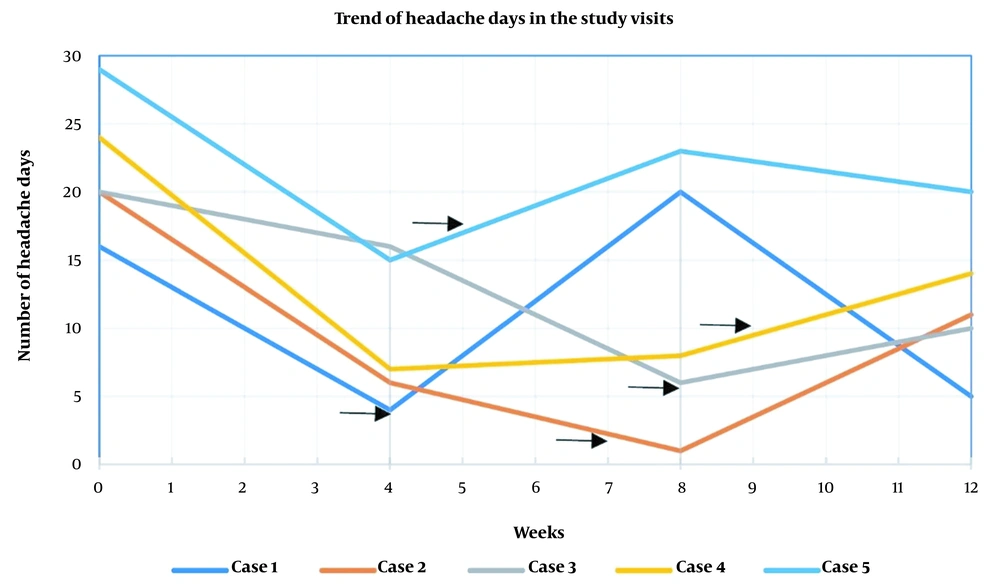
The first case was a 50-year-old woman diagnosed with CM without aura and medication overuse headache (MOH). As defined by the ICHD-3, MOH is a condition that occurs in patients with a pre-existing primary headache disorder, manifesting as a headache on 15 or more days per month for over three months, associated with 10 to 15 days of painkiller usage per month for at least 3 consecutive months (7). She also had a medical history of hypertension that was controlled with 25 mg of losartan twice daily. After the BTX-A injection, she initially showed a remarkable reduction in headache days, which surged from four to 20 days per month after the third dose of the Sinopharm COVID-19 vaccine, injected four weeks after the BTX-A injection. To manage the exacerbation of her headaches, a short duration of NSAID was initiated and then tapered off (100 mg celecoxib capsules were initiated every six hours for three days, decreased every three days to every eight hours, 12 hours, once daily, and then discontinued). The patient responded well, and her headache days decreased again (Table 2).
| Case Number | COVID Vaccine Injection Time (Weeks After Enrollment) | Headache Days per Month (Baseline) | Maximum Headache Intensity (Baseline) | Headache Days per Month (Visit 1, Week 4) | Maximum Headache Intensity (Visit 1) | Headache Days per Month (Visit 2, Week 8) | Maximum Headache Intensity (Visit 2) | Headache Days per Month (Visit 3, Week 12) | Maximum Headache Intensity (Visit 3) |
|---|---|---|---|---|---|---|---|---|---|
| Case one | 4 | 16 | 10 | 4 | 10 | 20 a | 10 a | 5 | 9 |
| Case two | 7 | 20 | 9 | 6 | 10 | 1 | 5 | 11 a | 8 a |
| Case three | 8 | 20 | 8 | 16 | 6 | 6 | 7 | 10 a | 6 a |
| Case four | 9 | 24 | 9 | 7 | 8 | 8 | 6 | 14 a | 7 a |
| Case five | 5 | 29 | 6 | 15 | 6 | 23 a | 8 a | 20 | 8 |
Headache Days per Month and Maximum Headache Intensity of the Cases in Each Visit. The Baseline Visit Shows the Number of Headache Days and Maximum Intensity of Headaches one Month Before Abobotulinum Toxin Injection.
The second case was a 31-year-old woman diagnosed with CM without aura with MOH and a medical history of gastroesophageal reflux disease and asthma. Her concomitant medications included 25 mg of amitriptyline every night, 50 mg of topiramate twice daily, and a salbutamol (albuterol) inhaler as needed. She showed a good response in the initial two months post-BTX-A injection. However, her headache days increased by 10 days after the third dose of the Sinopharm COVID-19 vaccine, injected in the seventh week of the study.
The third case was a 41-year-old woman diagnosed with CM without aura, with no medical history, except for taking a daily iron supplement for six months. She showed a favorable response in the second month following the BTX-A injection, but her headache days increased from six to 11 days after the third dose of the Sinopharm COVID-19 vaccine, injected in the eighth week of the study.
The fourth case was a 40-year-old woman diagnosed with CM without aura and MOH, without any medical history. Her only concomitant medication during the past year was 5 mg of zolpidem as needed. She initially responded well to the BTX-A injection, but her headache days increased from eight to 14 days after the fourth dose of the AstraZeneca COVID-19 vaccine, injected in the ninth week of the study.
Lastly, the fifth case was a 45-year-old woman diagnosed with CM without aura and anxiety as a comorbid condition. Her concomitant medications were 10 mg of nortriptyline nightly and 20 mg of citalopram daily in the past 6 months prior to botulinum toxin injection. She initially experienced a roughly 50% reduction in her headache days post-BTX-A injection, but her headaches worsened from 15 to 23 days after the second dose of the Sinopharm COVID-19 vaccine, which was injected five weeks after the BTX-A injection. In order to control the patient's headaches, we increased nortriptyline to 25 mg daily in the second study visit, and at the end of the study, topiramate was prescribed and titrated to a final dose of 50 mg twice daily. The trend of the patient's headache days per month is represented in Figure 1 and detailed in Table 2.
3. Discussion
The five cases presented in the current study experienced headaches worsening after vaccination, despite achieving a proper response to botulinum toxin. This finding might be contrary to an expert opinion that concluded COVID-19 vaccines and their produced antibodies do not lessen the efficacy of migraine preventive therapies, and no delay would be necessary regarding the timing of vaccination (8).
Worsening migraine headaches has been previously reported following COVID-19 vaccination (9). A retrospective cohort study assessed 45 patients and concluded that COVID-19 mRNA vaccination reduces the efficacy of botulinum toxin type A when administered for aesthetic indications (10). A case report presented two cases of sub-acute hypersensitivity reactions to botulinum toxin type A, injected for cosmetic purposes following COVID-19 vaccination, and recommended that botulinum toxin should be administered at least two to three months after COVID-19 vaccination (11).
Due to the limited number of cases and lack of understanding of the possible mechanism of COVID-19 vaccines that aggravate migraine headaches, no direct conclusions can be reached about whether these vaccines reduce BTX-A's efficacy. Previous studies have shown that the effectiveness of botulinum toxin gradually diminishes after two and a half to three months (12). This makes it particularly challenging to establish a connection between the worsening effect of COVID-19 vaccines on headaches in cases two to four, who received their vaccine two to three months after their toxin injection. Their relapse might have been due to the natural elimination of botulinum toxin's effect. On the other hand, a systematic review and meta-analysis showed that the onset of headache after COVID-19 vaccination was within the first day of injection and lasted four to 72 hours (3). Therefore, the increased number of headache days in these cases, which were all more than three days, could be due to the diminished efficacy of botulinum toxin caused by the vaccines. Further studies are suggested to discover the underlying mechanisms through which the possible decrease in efficacy occurs and to find the optimum time interval for these injections.
To our knowledge, this study is the first to examine possible interactions between COVID-19 vaccines and botulinum toxin in treating chronic migraines. This study's strength is attributed to its hypothesis on the temporal relationship between botulinum toxin administration and COVID-19 vaccination based on real-world observations. Nevertheless, the interpretation of results can be challenging due to the natural decrease in botulinum toxin's efficacy over time, which can coincide with increased headaches following vaccination. It is also possible that other confounding variables, such as stress, lifestyle changes, and medications, may have affected the outcome.
3.1. Conclusions
In the case of COVID-19 vaccination during the first month of administering botulinum toxin for the management of CM, there might be a possibility of a decrease in the efficacy of botulinum toxin, which should be explained to the patients. Therefore, the authors suggest postponing BTX-A injection by at least one month following COVID-19 vaccination.