1. Background
Insufficient production of insulin by the pancreas leads to type 1 diabetes (DM1), which is a chronic condition (1). In 2017, there were 451 million reported cases of diabetes worldwide, with a projected increase to 693 individuals with DM1 by 2045. Among the significant challenges faced by DM1 patients, an elevated risk of diabetic foot ulcers (DFUs) is prominent. DFUs arise from the interplay of several relevant factors, namely infection, ischemia, and neuropathy, which are the primary pathogenic causes of diabetic foot complications. Evidence suggests that lower limb amputation occurs in approximately 75% to 85% of cases subsequent to foot ulcers, which are commonly linked to chronic infection. These ulcers are characterized by reduced recruitment of endothelial progenitor cells (EPCs), impaired angiogenesis, decreased proliferation and migration of skin cells such as keratinocytes and fibroblasts, and heightened inflammatory cell presence (2).
In diabetes wounds, the parenchyma surrounding the wound, comprising fibroblasts, myofibroblasts, inflammatory cells, new blood vessels, regenerated neurons, and other cell types, undergoes detrimental changes (3). In response to tissue damage, fibroblast, endothelial, and macrophage cells, particularly endothelial cells (EC), secrete basic fibroblast growth factor (FGF-2), which plays a critical role in promoting wound healing (4, 5). Several studies have demonstrated the potential of FGF-2 in accelerating the healing process of diabetic wounds and its promise as a therapy for diabetic ulcers (4, 5).
Basic fibroblast growth factor (bFGF), also referred to as FGF2 plays a protective role by preventing endothelial cell death and promoting endothelial cell proliferation and angiogenesis. The signaling pathways involving FGF and microRNA (miRNA) have been demonstrated to influence various cellular processes, such as cell specification, proliferation, migration, differentiation, and survival (6).
MicroRNAs are non-coding RNAs that regulate gene expression.
Recent research indicates that miRNAs play a substantial role in both normal healing processes and the etiology of chronic ulcers, such as DFUs (7). Among the earliest mammalian miRNAs discovered, microRNA-21 (miR-21) has been found to participate in numerous biological activities. Increasing evidence suggests that miR-21 plays a crucial role in cutaneous injury and skin wound healing by forming intricate networks with its target genes and signaling pathways (8). The therapeutic effectiveness of miR-21 may be attributed to its ability to enhance fibroblast differentiation, promote angiogenesis, exert anti-inflammatory effects, enhance collagen synthesis, and facilitate wound re-epithelialization. Xie et al. discovered that miR-21 inhibits inflammation and promotes wound healing by modulating NF-κB expression through PDCD4 and FGF pathways (8). However, in diabetic wounds, the expression of miR-21 is significantly lower (8). Reduced miR-21 levels are also associated with increased expression of its target genes (9-11). As a result, increasing the levels of miRNA-21 in damaged tissue can effectively reduce inflammatory signals and enhance the healing process (11, 12). In essence, it is now acknowledged that over 100 molecules contribute to impaired skin healing in a non-conventional manner among individuals with diabetes (13). The use of appropriate medications and biomodulators appears to hold promise in the treatment of non-healing ulcers (14).
Mesenchymal stem cells (MSCs) have emerged as a valuable and effective treatment for skin wounds in contemporary medicine. Their remarkable differentiation potential, minimal immunogenicity, ease of collection, and significant contribution to the natural wound healing process make them an attractive therapeutic option.
Adipose tissue-derived mesenchymal stem cells (AD-MSCs) expedite the healing process of skin wounds through various mechanisms. These include promoting angiogenesis, granulation, and epithelialization, reducing inflammation, facilitating cell migration, and aiding in the reconstruction of the extracellular matrix (ECM) in tissues. The presence of AD-MSCs contributes to the establishment of favorable microenvironments for regenerative wound healing (15, 16). The extraction of adipose-derived stem cells (ADSCs) from adipose tissue and their subsequent in vitro expansion are straightforward processes. Preclinical investigations have demonstrated that ADSCs possess the ability to expedite the healing of wounds in diabetic patients. This is achieved through enhancements in epithelialization and granulation tissue formation, as well as the release of anti-apoptotic, anti-inflammatory, and angiogenic cytokines (17).
To the best of our knowledge, no previous studies have examined the impact of human adipose-derived stem cells (hADSCs) on the bacterial count, stereological parameters, and the expression of microRNA-21 and FGF2 in a model of infected, ischemic, and delayed wound healing in rats with DM1.
2. Objectives
Our study aimed to investigate the effects of hADSC administration on the bacterial count, stereological parameters, microRNA-21 expression, FGF2 expression, and wound size in a model of infected, ischemic, and delayed wound healing in rats with DM1.
3. Methods
3.1. Animals and Study Design
Twenty-four male Wistar rats, aged three months, were randomly divided into four groups (n = 6 per group). Type 1 diabetes was induced in all experimental animals. A model of delayed, ischemic, and MARS-infected wounds was created in all experimental animals. The experimental animals were allocated into four groups as follows: CGday4 and CGday8, which served as control groups 1 and 2, respectively. The hAGday4 group consisted of rats that received allograft hADSCs and were euthanized four days after surgery. The hAGday8 group received hADSCs and were euthanized on day eight after surgery.
In this study, we evaluated the wound area, microbial colony counts, stereological examination, and the expression of FGF2 and miR-21 on days 4 (inflammation phase) and 8 (proliferation phase), respectively.
The experiment was approved by the Medical Ethics Department of the School of Medicine, Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran (file no. IR.SBMU.MSP.REC.1400.295).
3.2. Separation, Expansion, and Immunophenotyping of Allograft h-ADS
Human adipose tissue was obtained from the subcutaneous tissue of a healthy 38-year-old woman who underwent mammoplasty surgery, following informed consent from the patient. Approximately 5 cc of adipose tissue was manually crushed and subsequently washed in PBS containing penicillin and streptomycin. The tissue was then digested using a 0.1% collagenase-I solution for 40 - 60 minutes at 37°C, followed by centrifugation. Red blood cells were removed using a lysis buffer. The cell pellets were then resuspended in Dulbecco’s modified eagle medium (DMEM) supplemented with 20% fetal bovine serum (FBS) and transferred into T-75 flasks containing DMEM supplemented with 20% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The expression of MSC markers in ADSs was evaluated using flow cytometry, following the previously described methods (18, 19).
3.3. ADS Transplantation
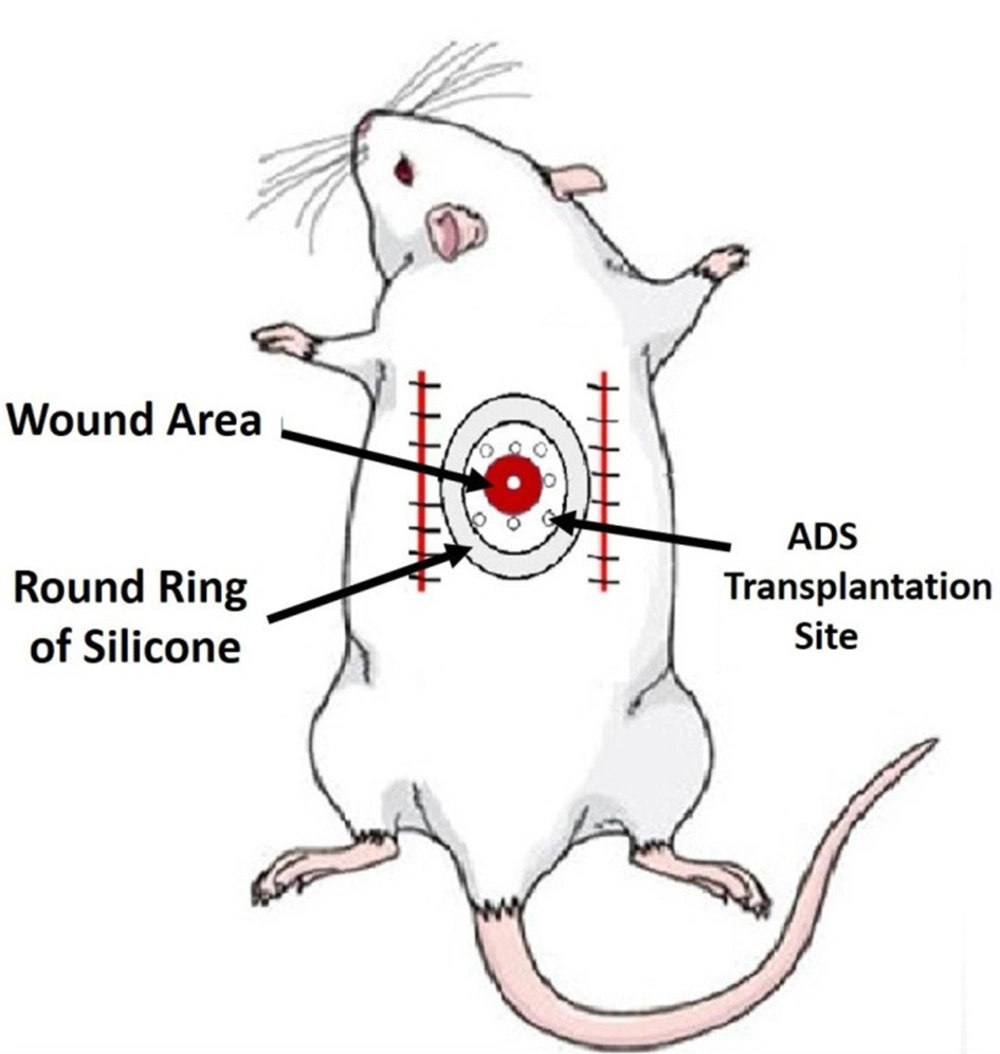
A total of 1 × 106 ADSs from passage four were intradermally transplanted into eight areas surrounding the ulcer, approximately 4 - 5 mm from the edge of the ulcer (Figure 1).
3.4. DM1 Induction
DM1 was induced using a single intraperitoneal injection of streptozotocin (STZ) at a dose of 40 mg/kg (20). One week after the STZ injection, blood samples were collected to measure blood sugar levels. DM1 was defined as blood sugar levels exceeding 250 mg/dL. All rats with DM1 were monitored for 30 days to confirm the induction of DM1 (20).
3.5. Clinical Tests
The body weight and blood glucose levels of DM1 rats were monitored throughout the experiment.
3.6. Surgery
Rats were anesthetized using 50 mg/kg of ketamine and 5 mg/kg of xylazine. Prior to surgery, all treated rats received ceftriaxone at a dose of 50 mg/kg, which was continued for 24 and 48 hours post-surgery. A dorsal, bipedicle skin flap measuring 10 × 3 × 5 cm was created on the dorsal skin of all rats. A 12-mm full-thickness excisional round ulcer was then generated at the midpoint of the flap using a biopsy punch. A round piece of silicone was sutured using a 0.4 silk skin holder to secure the skin lesion. Additionally, all experimental rats were administered 20 mg/kg of ibuprofen every 8 - 12 hours before and for five days after surgery (Figure 1).
3.7. Injection of MRSA into the Ulcer Area and Microbiological Test
In this study, the MRSA strain (ATCC 25923) was utilized. The methodology for this strain has been previously described. In summary, a colony of MRSA with a concentration of 2 × 108 at 1 cc was prepared. A 100-l aliquot (2 × 107 MRSA) was locally injected into all ulcers following surgery.
For microbiological tests, samples were collected from the injured areas on days 0, 4, and 8, respectively. The bacterial colonies were then counted as the number of bacteria per sample (colony-forming units (CFUs)) (21, 22).
3.8. Wound Area Measurement (WAM)
In this study, a digital camera was used to capture photographs of the wound site on days 0, 4, and 8. The size of the wounds (measured in mm2) was then determined using Image J-NIH software (USA) and compared to the measurements taken on day zero (23).
3.9. Wound Strength Examination
On day 16, a 50 mm × 5 mm sample was collected from each wound site and placed in a material testing machine. The rate of deformation was set at 10 mm/min. The maximal force (N) and stress under high load (N/cm2) of the samples were determined from the load-deformation curve (21).
3.10. Histological and Stereological Analysis
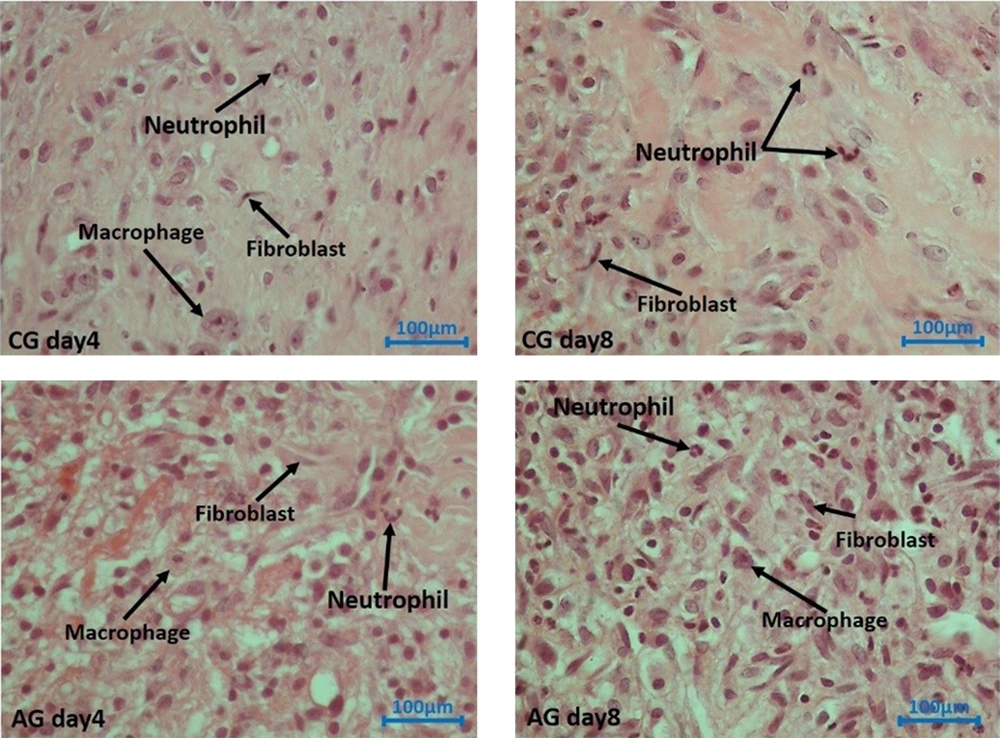
The skin samples obtained from the wound areas were initially fixed in formalin solutions. Paraffin-embedded tissue blocks were prepared, and serial sections of 5-μm thickness were cut. These sections were then stained using a standard laboratory staining method. The numerical density (Nv) of neutrophils, macrophages, fibroblasts, and blood vessels in each wound area was subsequently calculated.
Cell count estimation:
ΣQ = the number of cells; h = dissector height; a/f = the entire area of the counted frames; and ΣP = the total number of counting frames.
Number of blood vessels:
where ΣQ = all vessel numbers counted per wound. The number of blood vessels was considered a marker for angiogenesis.
3.11. RNA Extraction and Quantitative Real-time Polymerase Chain Reaction Detection of miR-21 Expression Level
Initially, skin wound samples were obtained from the designated areas on the rats. Subsequently, total RNA was extracted from these skin samples using Trizol (Invitrogen, Carlsbad, CA).
The obtained total mRNA was assessed for purity using a Bioanalyzer 2100 (Agilent Technologies, Inc., Santa Clara, CA) by measuring the absorbance ratio at 260/280 nm. Subsequently, the purified total mRNAs were stored in a nitrogen tank at -80°C. For reverse transcription, cDNA reverse transcription kits (Applied Biosystems, Carlsbad, CA) were used.
After pre-amplification, the expression levels of the target genes were evaluated using SYBR Green and quantitative real-time polymerase chain reaction (qRT-PCR) on a StepOne™ thermal cycler (Applied Biosystems). GAPDH was used as a housekeeping gene, and RNU6b (RNA, U6 small nuclear 2) served as a reference gene. The primer lists for GAPDH, FGF2, microRNA21, and RNU6b can be found in Table 1. The gene expression level in the studied rats was calculated using the REST 2009 software, employing the Pair Wise Fixed Reallocation Randomization Test. The obtained data were analyzed using the ΔΔCT and Pfaffl methods for comparison.
| Target Gene | Primer Sequence | Tm |
|---|---|---|
| FGF2 | ||
| F | GACCCACACGTCAAACTACA | 57.77 |
| R | GCCGTCCATCTTCCTTCATAG | 58.24 |
| GAPDH | ||
| F | ATCTGACATGCCGCCTGGAG | 61.40 |
| R | AAGGTTGGAAGAATGGGAGTTGC | 60.25 |
| microRNA-21 | ||
| F | CGCCCGTAGCTTATCAGA | 62.10 |
| PROB | TGCATACGACTCAACATC | 59.14 |
| RNU6 small nuclear 2 | ||
| F | GTGCTCGCTTCGGCAGCACATAT | 64.40 |
| PROB | TGTATCGTTCCAATTT TATCGGATGT | 63.05 |
Abbreviations: FGF2, fibroblast growth factor 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; RNU6, RNA, U6 small nuclear 2.
3.12. Statistical Analysis
The collected data were analyzed using SPSS version 21, and the results were reported as the mean ± standard deviation (SD). Statistical analysis of the data obtained from body weight measurements, microbiological tests, wound strength tests, and wound area size involved the use of t-tests, one-way analysis of variance (ANOVA), and the least significant difference (LSD) test. A significance level of P < 0.05 was considered statistically significant.
4. Results
4.1. The Expression of the Stem Cell Marker
The results obtained from flow cytometry analysis revealed that ADSs expressed CD11b (0.21%) and CD45 (0.66%). Furthermore, CD44 and CD105 were found to be fully expressed (100%) by ADSs (Figure 2).
4.2. Clinical Observations
Clinical evidence revealed that all rats exhibited DM1, as indicated by a significant increase in blood sugar levels and a decrease in body weight following STZ treatment (Table 2). The results of our independent t-test indicated a significantly higher body weight in the hAG groups compared to the respective control group (P = 0.005).
a Values are expressed as mean ± SD.
b P < 0.01
c P < 0.05
The hAG group showed a significant improvement in blood sugar levels, with a more pronounced decrease compared to the control group.
4.3. Microbial Findings
4.3.1. Day 4
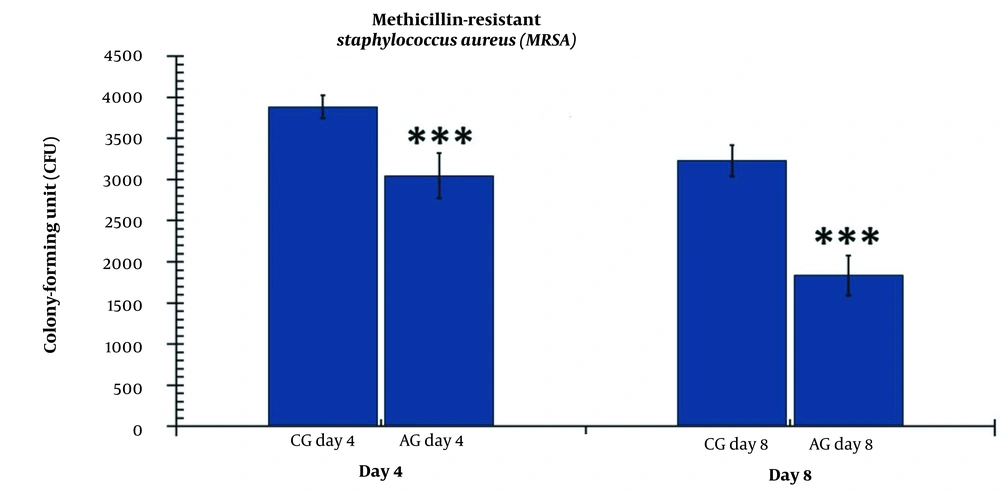
Our microbial study showed that the level of colony-forming units (CFUs) was significantly lower in the hAGday4 group (P = 0.000) compared to the CGday4 group (Figure 3). Similarly, there were significant changes in the CFU count between the hAGday8 group and the CGday8 group (P = 0.004).
The number of colony-forming units (CFUs) in the experimental and control groups on days 4 and 8. The independent-samples t-test and least significant difference (LSD) tests were used to examine the results, which were shown as a mean ± SD. * refers to P < 0.05, ** refers to P < 0.01, and *** refers to P < 0.001. CG, control group; AG, human adipose-derived stem cell group.
The changes in the CFU count were significantly greater in the hAGday8 group compared to the hAGday4 group (P = 0.001) (Figure 3).
4.4. Wound Area Measurement (WAM)
4.4.1. Day 4
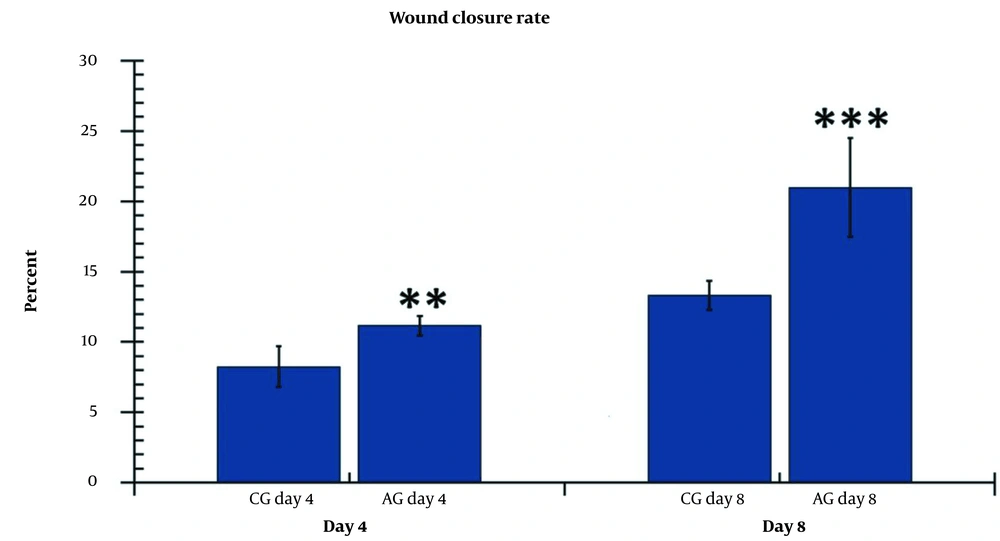
On days 4 and 5, the wound area in the AGday4 experimental group was significantly larger than that in the CGday4 control group (P = 0.009) (Figure 4).
4.4.2. Day 8
The wound area size was significantly smaller in the hAGday8 groups (P = 0.001) compared to the CGday8 groups. The results in the hAGday8 groups exhibited a greater reduction in wound area compared to the hAGday4 group (P = 0.021) (Figure 4).
4.5. Tensiometric Examination
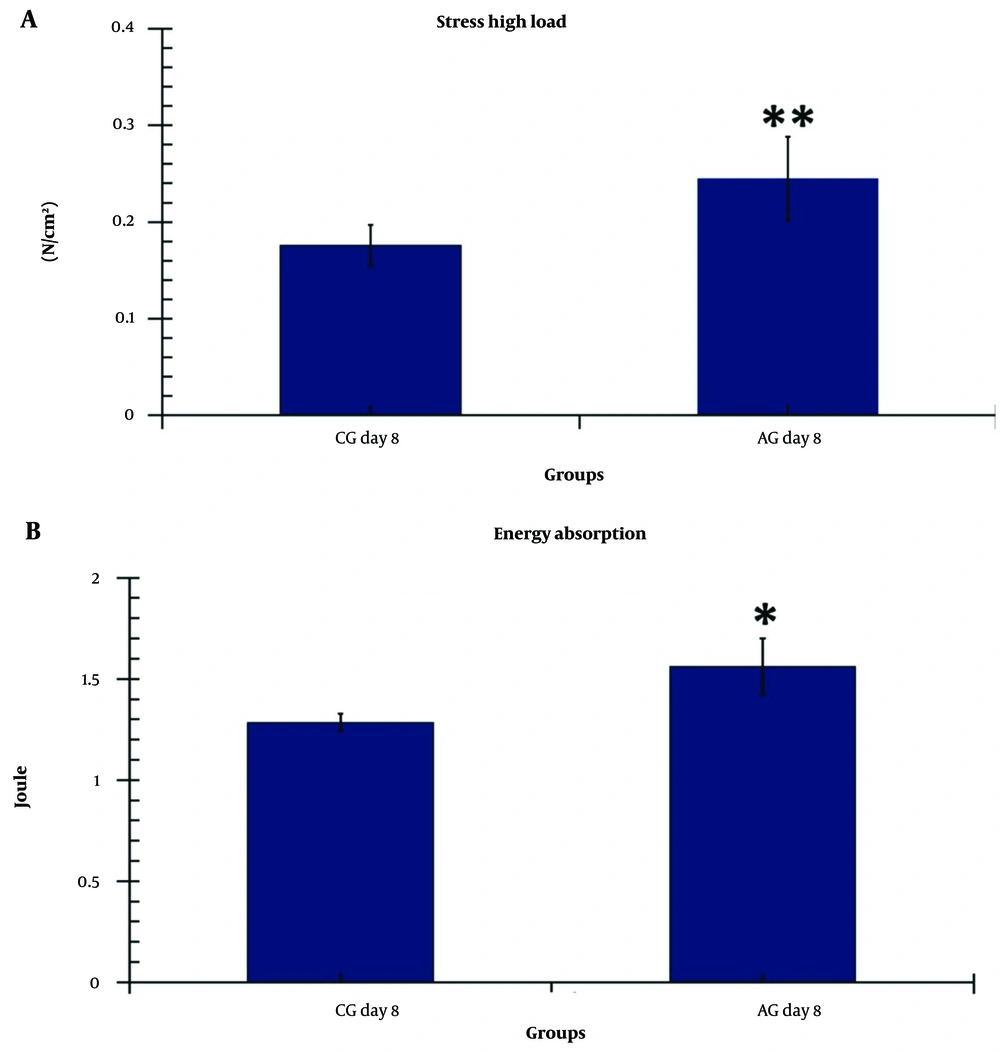
All P-values were correlated with the LSD test. Tensiometric examination results are shown in Figure 5.
The level of stress high load in the treatment group (AG) was significantly higher than in the control group (P = 0.01) (Figure 5A). Additionally, the level of maximum energy absorption in the AG group was significantly improved compared to the CG (P = 0.04) (Figure 4B).
4.6. Stereological Findings
Figures 6 - 8 display the stereological findings for all studied groups on days 4 and 8. In terms of the stereological study, it was observed that on both days 4 and 8, all experimental groups exhibited superior stereological results compared to the control group. Furthermore, significant improvements were observed in the hAGday8 treatment group compared to the hAGday4 group (P < 0.05).
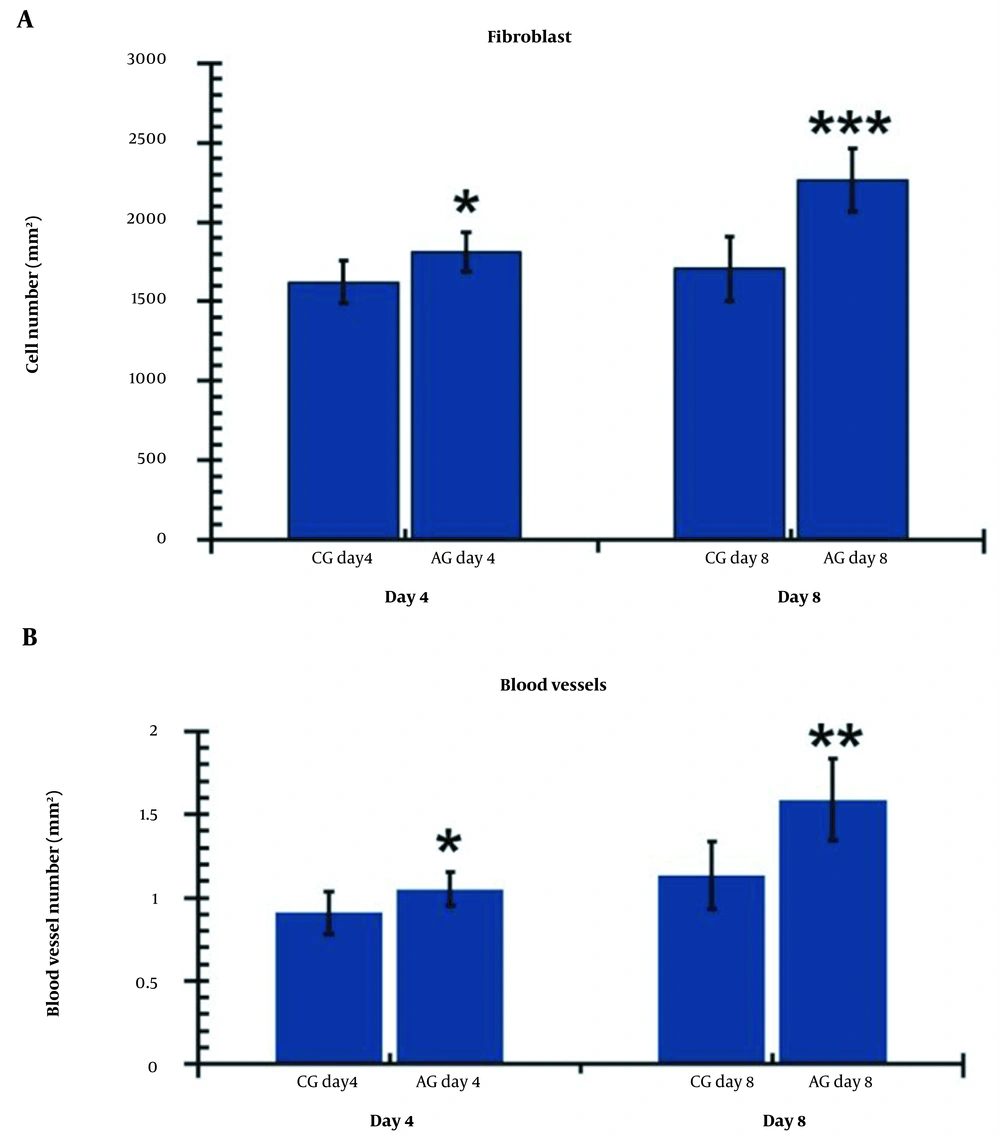
A, Comparison of the wounds’ fibroblasts; and B, Blood vessels numbers on days 4 and 8 in the research groups. The independent-samples t-test and least significant difference (LSD) tests were used to examine the results, which were shown as a mean ± SD. * P < 0.05 5; ** P < 0.01; *** P < 0.001. CG, control group; AG, human adipose-derived stem cell group.
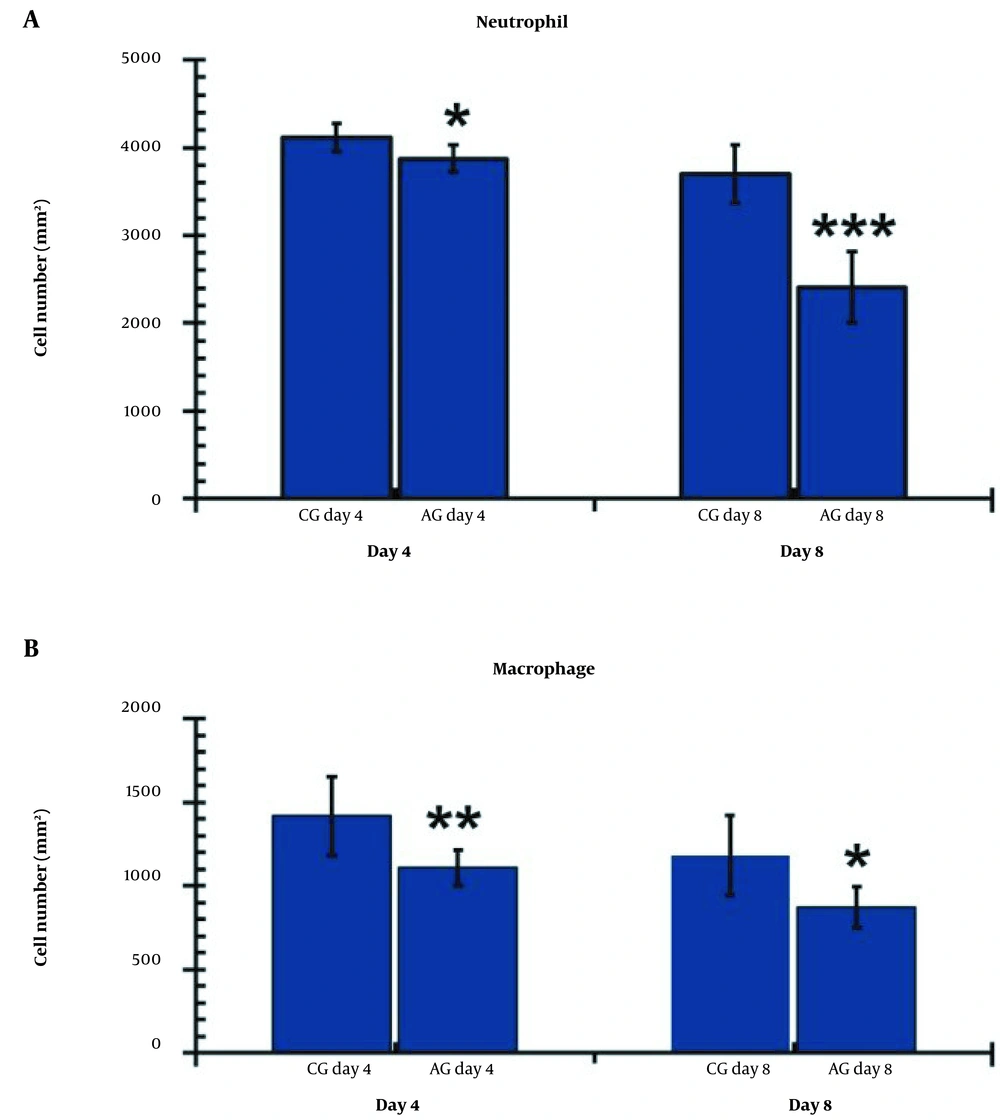
A, Comparison of the wounds’ neutrophil; and B, Macrophage numbers on days 4 and 8 in the research groups. The independent-samples t-test and least significant difference (LSD) tests were used to examine the results, which were shown as a mean ± SD. * P < 0.05; ** P < 0.01; *** P < 0.001. CG, control group; AG, human adipose-derived stem cell group.
4.7. Fibroblast Count
Figure 7A illustrates the fibroblast findings in the studied groups on days 4 and 8. On both days 4 and 8, the experimental groups exhibited a higher number of fibroblasts compared to the control group (P = 0.016 for day four and P = 0.000 for day 8). Additionally, a significant increase in fibroblasts was observed in the hAGday8 treatment group compared to the hAGday4 group (P = 0.017).
4.8. Angiogenesis
Figure 7B demonstrates that the hAGday4 and hAGday8 groups had significantly more blood vessels than the CGday4 and CGday8 groups on day 4 (P = 0.000, P = 0.003) and day 8 (P = 0.041, P = 0.003), respectively. Additionally, the number of blood vessels in the CGday8 group was significantly lower compared to the hAGday8 group (P = 0.037).
4.9. Neutrophils Count
Figure 8A illustrates the neutrophil findings in the studied groups on days 4 and 8. On both days 4 and 8, the experimental groups exhibited significantly lower levels of neutrophils compared to the control group (P = 0.013 for day four and P = 0.000 for day 8). Furthermore, we observed fewer neutrophils in the hAGday8 treatments compared to hAGday4 (P = 0.01).
4.10. Macrophage Count
Figure 8B illustrates the macrophage findings in the studied groups on days 4 and 8. On both days 4 and 8, the experimental groups exhibited significantly lower levels of macrophages compared to the control group (P = 0.008 for day four and P = 0.010 for day 8).
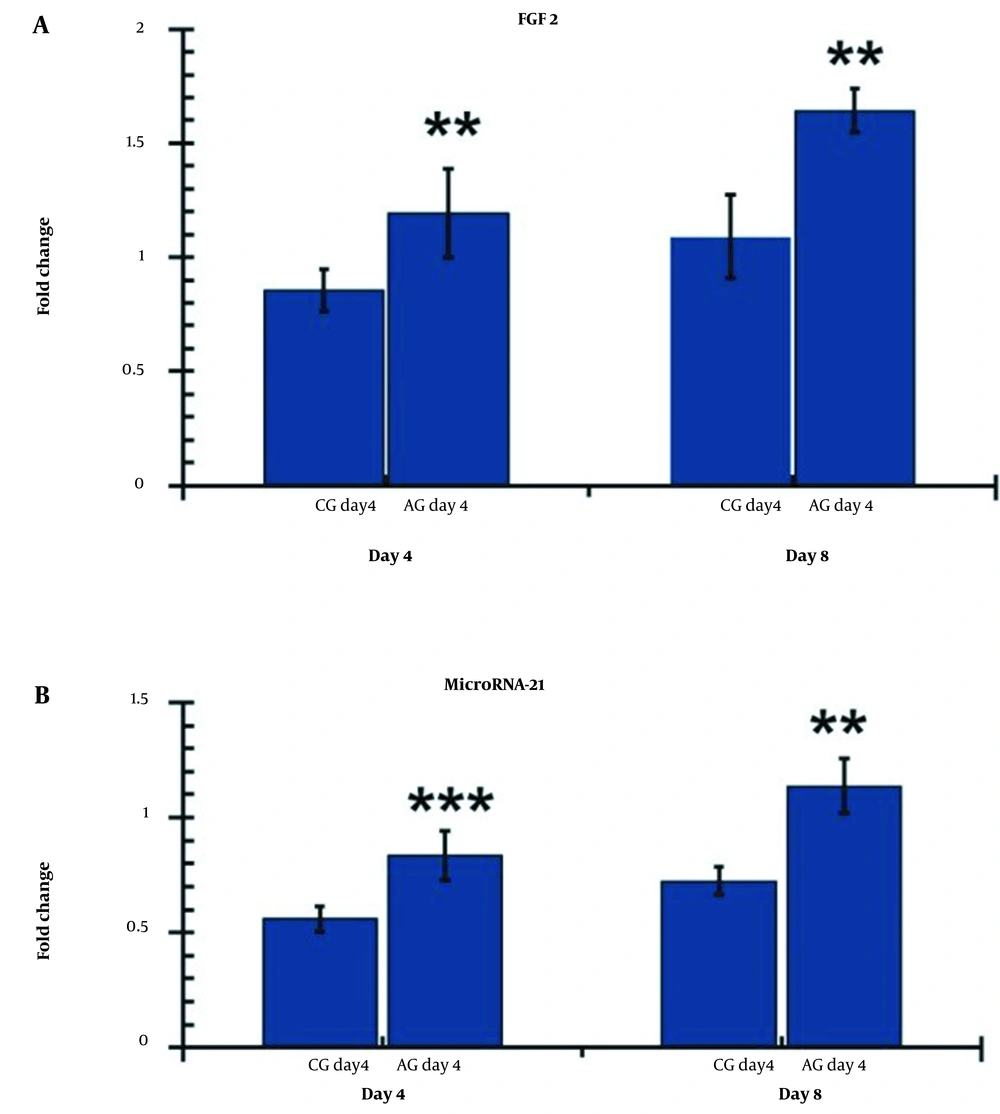
4.11. The Expression Level of FGF2
On day 4, the CGday4 groups exhibited significantly higher levels of FGF2 (P = 0.008) compared to the control group (P = 0.024). Furthermore, the hAGday8 group demonstrated higher levels of FGF2 compared to the CGday8 group (P = 0.002), as shown in Figure 9A.
Comparison of FGF2 and miR-21 levels on days 4 and 8 in the damaged areas of the research groups. The independent-sample t-test and least significant difference (LSD) tests were used to evaluate the data, which was presented as mean standard deviation. ** P < 0.001; *** P < 0.001. CG, control group; AG, human adipose-derived stem cell group.
4.12. The Gene Expression Level of miR-21
Figure 9B displays the gene expression levels of miR-21 in the wound bed on days 4 and 8.
On day 4, the levels of miR-21 in the hAGday4 group were significantly higher than in the control group (P = 0.007). On day 8, the level of miR-21 in the hAGday8 group was also significantly higher than in the control group (P = 0.003). Moreover, the findings of the hAGday8 group were significantly more pronounced than those of hAGday4 (P = 0.010).
5. Discussion
In this study, we aimed to assess the therapeutic effects of hADS on the bacterial count, stereological parameters, expression of microRNA-21, FGF2, and ulcer size in a rat model of infected, ischemic, and delayed wound healing in DM1. Overall, our findings demonstrate that both experimental groups (hAGday4 and hAGday8) exhibited substantial reductions in microbial flora counts, improved wound closure rates, and increased expression of microRNA-21 and FGF2 on days 4 and 8. Additionally, the hAGday8 group showed significantly more pronounced effects on stereological parameters compared to the other groups.
There are numerous promising advancements in cell therapy and tissue engineering, offering potential therapeutic options for addressing the challenges associated with impaired skin healing, including DFUs (22, 24).
In recent years, stem cell therapy has emerged as a novel therapeutic approach for addressing various conditions, including impaired wound healing and tissue regeneration. Several types of stem cells, including ADSCs, bone marrow-derived mesenchymal stem cells (BM-MSCs), endothelial progenitor cells, keratinocytes, and human skin fibroblasts, have been utilized in both clinical and preclinical settings to enhance wound repair. Among these, mesenchymal ADSCs have gained significant attention due to their abundance and demonstrated efficacy in promoting wound healing.
Due to their abundant adipose tissue content, extensive ex vivo proliferative capacity, ease of isolation, and ability to secrete pro-angiogenic growth factors while minimizing immunological reactions, adipose tissue cells have emerged as a readily accessible and preferred cell source for therapeutic applications in the management of chronic non-healing wounds (25-27). Transplantation of ADSCs has enhanced neovascularization and improved blood flow in ischemic tissues in murine models (28, 29).
Additionally, studies have demonstrated that ADSCs contribute to arteriogenesis in ischemic tissue through paracrine signaling pathways facilitated by the secretion of growth factors (26, 28). It is hypothesized that ADSs may play a direct role in wound regeneration by replacing damaged cells via differentiation into epidermal cells (30). Furthermore, the findings from two relevant studies have revealed that engrafted ADSs express endothelial markers, promoting the formation of vascular networks in ischemic organs (31, 32).
In our study, we observed a significant reduction in wound area following the hADS injection, accompanied by an increase in the rate of wound closure. Notably, the administration of hADS exhibited an enhanced bactericidal effect and improved wound strength. These findings highlight the potential of ADS as an anti-inflammatory agent and support the use of ADS-based therapy in the treatment of DFUs.
Bacterial infections have been identified as the primary causative factor contributing to impaired wound healing (33). MRSA, a commonly encountered strain of staphylococcal bacteria, is responsible for numerous wound infections and has shown an alarming increase in antibiotic resistance (34). This rise and adaptation of MRSA in DFU areas (34) have resulted in an expanding spectrum of untreatable staphylococcal infections (35). Consequently, there is an urgent need for innovative approaches to combat MRSA infections and address antimicrobial resistance (AMR) concerns (36). The focus of our study was specifically to evaluate the antibacterial effects of ADS (36), as they have demonstrated antimicrobial properties in animal models.
In this study, we demonstrated that the use of ADSs can significantly decrease the microbial count compared to the control group. These findings are consistent with Lipovsky et al.’s laboratory experiment, which showed that ADS inhibits Staphylococcus aureus effects by generating ROS (37). The antibacterial effects of ADSs may be attributed to the induction of ROS. Kouhkheil et al. investigated the effects of CM-hBMMSC (four injections) and photobiomodulation (890 nm, 0.2 J/cm2, 80 Hz) alone and in combination on wound strength and CFU in an MRSA-infected wound model in DM1 rats (22).
Kouhkheil et al. demonstrated that CM-hBMMSC and PBMT, either alone or in combination, significantly reduced CFUs compared to the control group (22). In another study by Fridoni et al., the individual and combined effects of CM-hBMMSC and PBMT on stereological parameters were evaluated in an MRSA-infected wound model in rats with DM1 (38). The researchers concluded that the simultaneous use of PBMT and CM-hBMMSC had anti-inflammatory and neo-vascular effects, promoting accelerated healing of skin damage in the MRSA-infected wound model in DM1 rats (38).
DFUs are characterized by a persistent inflammatory state marked by the accumulation of inflammatory cells, pro-inflammatory cytokines, and proteases. Consistent with this, our study observed increased numbers of neutrophils and macrophages in the control group on days 4 and 8.
A study conducted by Kim et al. reported that the transition from the inflammation phase to the tissue restoration phase is crucial for effective tissue repair and renewal of ECM (39). They also highlighted the pivotal roles of endothelial and fibroblast cells in ECM remodeling and angiogenesis, which are essential for proper wound closure (39). Consistent with the findings of Kim et al., our results also demonstrated that all experimental groups had significantly higher counts of blood vessels and fibroblast cells compared to the control group, indicating enhanced angiogenesis and fibroblast activity in the treated groups (39).
In our study, the use of ADS alone demonstrated a significant increase in the levels of fibroblasts and blood vessels compared to the other experimental groups. These findings suggest that ADS treatment can effectively enhance the stereological parameters and FGF levels within ECM during the proliferative and inflammatory phases of the injury-repairing process in an infected, ischemic, and delayed wound healing model in rats with TIDM. Furthermore, the results of the stereological and FGF examinations specifically conducted in the hADday8 group showed superior outcomes compared to the other groups.
FGF2 plays a crucial role in promoting endothelial cell proliferation and angiogenesis while also exerting a protective effect on endothelial cell survival. The signaling pathways involving FGF and miRNAs have been implicated in regulating various cellular processes, such as cell specification, proliferation, migration, differentiation, and survival (6).
One notable finding of our study is the expression levels of miR-21 and FGF2 genes. We observed significantly higher expression levels of miR-21 in all experimental groups compared to the control group, particularly during the proliferative phases. These results indicate that our experimental groups, especially the hADday8 group, exhibited favorable performance in terms of miR-21 gene expression.
Increased expression of miR-21 increases the proliferative response, decreases the inflammatory response, and increases wound healing (40).
The administration of ADS has been shown to enhance fibroblast proliferation, increase FGF2 expression, reduce inflammation in the wound bed, and promote the transition of IIDHWM to the proliferative and remodeling phases of wound healing. These findings are consistent with both our current study and previous research. In the treatment groups, ADS treatment resulted in a significant increase in granulation tissue formation (new dermal volume) during the proliferative phase of wound healing. Furthermore, it improved tensile strength and accelerated wound closure rate during the remodeling phase (41). Based on the findings from our control group, it is evident that there is dysregulated regulation of both the inflammatory and proliferative phases in diabetic skin. This dysregulation is characterized by elevated neutrophil and macrophage counts, as well as reduced levels of miR-21 and FGF2 in the wound bed (42-44). Our research indicates that the reduced expression of miR-21 and FGF2 in the control group, which was subsequently increased by hADS therapies, may be linked to the inherent dysregulation of inflammation, proliferation, and remodeling in this group. These findings align with several relevant studies that have demonstrated the critical role of miR-21 in wound healing, as it forms a complex network with its target genes (such as PTEN, RECK, SPRY1/2, NF-B, and TIMP3) and cascaded signaling pathways (such as MAPK/ERK, PI3K/Akt, Wnt/-catenin/MMP-7, and TGF-/Smad7-Smad2/3).
The therapeutic efficacy of miR-21 may be linked to several factors, including stimulation of fibroblast differentiation, improvement of angiogenesis, anti-inflammatory effects, augmentation of collagen production, and re-epithelialization of the wound (8, 44). Xie et al. reported that miRNA-21 exerts anti-inflammatory actions and improves wound healing by regulating the expression of NF-κB via PDCD4 (8).
However, in diabetic wounds, there is a significant decline in the expression of miR-21 (8, 45, 46). The reduced levels of miR-21 are closely associated with an amplified expression level of its target gene. Therefore, increasing the level of miRNA-21 in damaged tissue can help reduce inflammatory signals and improve the healing process (45).
5.1. Conclusions
ADS-based therapies improved the inflammatory and proliferative phases of wound healing in TIDM1 rats by enhancing stereological parameters such as fibroblast cells and blood vessels, decreasing microbial counts and inflammatory elements such as neutrophils and macrophages, and increasing the expression of miRNA-21 and FGF2.