1. Background
Streptococcus agalactiae, also known as group B Streptococcus (GBS) is a major cause of serious life threatening infections including sepsis, pneumonia and meningitis in neonates and young infants as well as other serious infections in women at gestational and postpartum period, individuals with diabetes, immunocompromised patients or adults and the elderly. Mortality rates due to GBS infection in newborns is 4% to 6% and higher in premature infants (1, 2).
Group B Streptococcus is classified to 13 variants (serotypes), based on recognized capsular polysaccharide (CPS) antigens (capsular polysaccharide synthesis cps gene cluster), from which nine serotypes, i.e. Ia, Ib, and II-VIII, are considered clinically important. Based on reported studies from the USA and Europe, it has been shown that serotypes Ia, II, III and V, are the main causes of human GBS disease, as these serotypes have been found in 80% to 90% of clinical isolates (3-5).
Capsular polysaccharide typing is significant and essential for epidemiological studies of GBS, pathogenesis and also other studies association with GBS infections, including surveillance programs and vaccine development in future. In fact, many attempts have focused on using CPS as immunoprophylactic antigens (4).
According to investigations, it has been found that type III often appears in neonatal disease followed by types Ia, Ib, II and V. In addition, these antigens are the cause of 96% and 88% of neonatal and adult diseases, respectively; therefore, a possible CPS vaccine must include these mentioned capsular antigens (6).
To ensure efficient development of vaccine, surveying worldwide distribution pattern of frequent serotypes will be important, in order to ensure involvement of the most appropriate bacterial components in one global GBS vaccine. Moreover, prevalence of GBS serotypes is age dependent and different between colonizing and invasive strains (7, 8). Typing of bacteria is often required when two or several samples (strains) are suspected to have epidemiological relationship, for instance, in nosocomial or foodborne outbreaks. Another situation, in which typing is required can be epidemiological surveillance of one infectious disease after a certain period of time in order to follow disease development and design possible infection control approaches. Another application of typing methods can be comparison among strains of bacterial species isolated from one patient for differentiating pathogenic strains from nonpathogenic ones (7-9).
Conventionally, GBS capsular typing is performed by serotyping (immunological) methods (10), however, complicated interpretation, high costs, and commercial availability of reagents for only a subset of serotypes are a limitation of these methods. Moreover, due to possible capsule operon mutations or rearrangements and the fact that encapsulation levels of GBS is different among strains particularly under experimental conditions, typing of such strains using immunological methods may fail or be difficult. Additionally, subjects such as those examining whether strains express capsular polysaccharide related genes (non-encapsulated) or express polysaccharide variants that fail to react with used antiserums, are non-distinguishable using these methodologies (11).
Although appropriate phenotypic methods can be used for outbreak isolates during a short period of time, yet such methods are generally not adequate for evolutional studies, and it has been known that these methods lack adequate capability of discriminatory (9).
Polymerase chain reaction (PCR)-based typing has been demonstrated that non-typeable strains resulted from immunological methods usually harbor the genetic information for synthesis these polysaccharides and consequently are typeable by PCR assay; in fact, molecular methods such as PCR assay offer more accurate and reliable typing of bacteria than phenotypic methods (12). On the other hand, in case of mutations in capsule synthesis genes, the molecular typing methods result in non-typeable strains (13); thereby CPS genotyping can be designed using both single PCR and/or multiplex PCRs (14-17).
2. Objectives
With regards to the mentioned issues, the aim of the present study was to determine capsular polysaccharide serotypes of clinical isolated GBS using multiplex PCR assay in Hamedan, for obtaining accurate information about distribution of GBS serotypes in this region.
3. Methods
3.1. Collection and Identification of Isolates
In this experimental study, a total of 62 GBS clinical strains (56 and 6 samples from female and male individuals, respectively) including vaginal swabs (n = 16, one sample from a non-pregnant woman and fifteen samples from pregnant women isolated from 203 collected specimens), urine cultures (n = 45), and blood culture (n = 1) isolates were collected from educational hospitals and private centers during nine months from June 2013 to February 2014 in Hamedan, Iran. All samples from male individuals were urine cultures. The study was done after obtaining informed consent from the participants and approval from the Hamadan University of medical science ethical committee with the following code: IR.UMSHA.REC.9304171965. The included individuals were women at 35 - 37 weeks of pregnancy with no clinical problems, referred to prenatal care of Fatemieh hospital and/or private centers in Hamadan. The criterion for exclusion was being a pregnant woman at < 35 weeks of gestation. Information about age for each collected sample was recorded. Based on the center for disease control and prevention (CDC) guidelines (1), processing of the vaginal swabs were performed as follows: specimen swabs were inoculated in Lim broth (Pronadisa Co, Spain) as a selective and enriched medium and incubated at 35 to 37°C in 5% CO2 (in candle jar) for 18 to 24 hours, then subcultured on Trypticase Soy agar (TSA) (Merck Co, Germany) with 5% blood and incubated at 35 to 37°C in 5% CO2 for 18 to 24 hours. Conventional phenotypic methods including gram staining, catalase test, sodium hippurate hydrolysis, bile esculin agar test, and CAMP reaction were used for microbiological presumptive identification of the isolates. The presumptive identified isolates were subcultured on Trypticase Soy agar with 5% blood and incubated at 35 to 37°C in 5% CO2 for 18 to 24 hours for obtaining single pure GBS colonies. Then these isolates were inoculated in brain heart infusion (BHI) broth (Merck Co, Germany) containing glycerol and blood and preserved at deep freeze (-70°C) conditions until further use.
3.2. DNA Extraction From Isolates
DNA was extracted from isolates using alkaline lysis method using the following procedure: three or four colonies from overnight cultured isolate were suspended in a sterile microtube containing 60 μL of lysis buffer (0.05 N NaOH, 0.25% sodium dodecyl sulfate-SDS) then vortexed and heated at 95°C for 15 minutes. Afterwards, 540 μL of TE buffer (50 mM Tris HCl, 1 mM EDTA, pH 8) was added to the microtube for diluting the obtained cell lysate. Subsequently, the microtube was centrifuged at 15000 rpm for five minutes to sediment cell debris. The supernatant was transferred to a new sterile microtube and used for PCR assay or frozen at -20°C until further use (17).
3.3. Confirmation of Identified Isolates as Group B Streptococcus Using Polymerase Chain Reaction
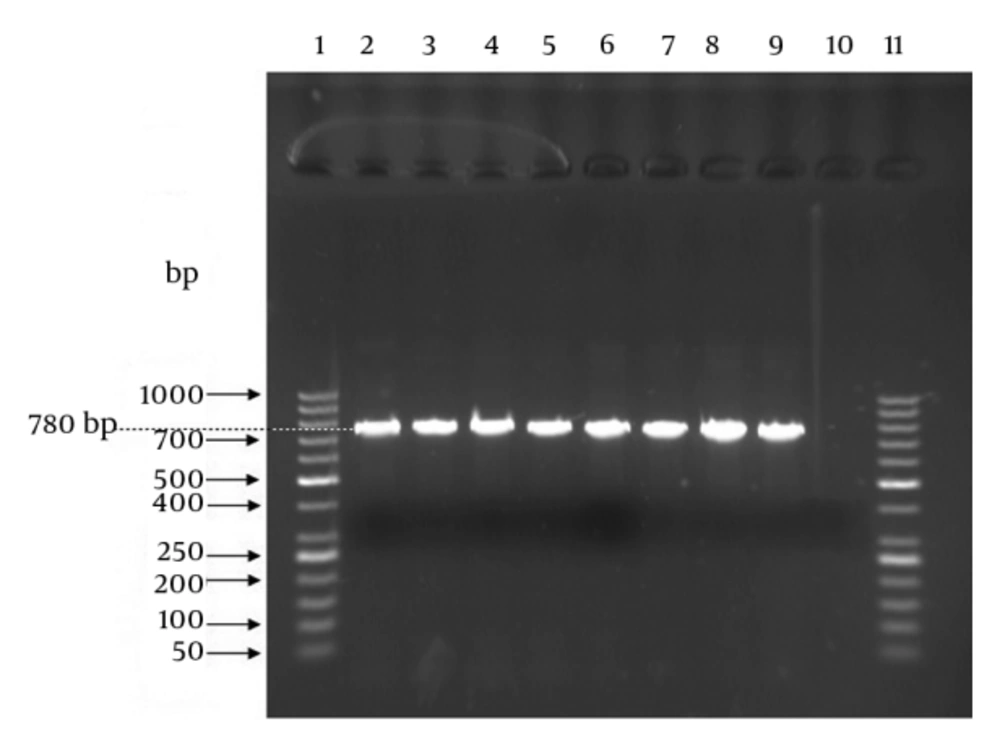
The PCR assay targeting the 780-bp atr gene (GenBank accession number: AF15135) specific for S. agalactiae species as internal positive control was performed for confirmation or definitive identification of the isolates. The forward and reverse primer sequences were CAA CGA TTC TCT CAG CTT TGT TAA and TAA GAA ATC TCT TGT GCG GAT TTC, respectively (8). The PCR reaction volume was 20 μL including 2 μL of bacterial DNA, 1 μL of forward primer, 1 μL of reverse primer, 10 μL of 2x Taq Premix-Master mix (Parstous Biotech Co, Iran), and 6 μL of sterile double distilled water. Amplification thermal cycles were as follows: an initial denaturation step for five minutes at 94°C followed by 35 cycles at 94°C for 30 seconds, 55°C for 55 seconds, and 72°C for one minute and a final extension cycle at 72°C for 10 minutes using Bio-Rad Thermal Cycler. For positive and negative controls, the S. agalactiae ATCC 12386 and Enterococcus faecalis ATCC 29212 were used, respectively. The PCR products and 50-bp DNA size marker (Fermentase Co, USA) were run simultaneously on 1.5% agarose gel stained with DNA safe stain (SinaClon Co, Iran) at 80 V for one hour. Finally, the agarose gel was visualized and photographed using UV transilluminator (Vilbert Lourmat Co, Japan).
3.4. Molecular Serotyping of Group B Streptococcus Isolates Using Multiplex Polymerase Chain Reaction
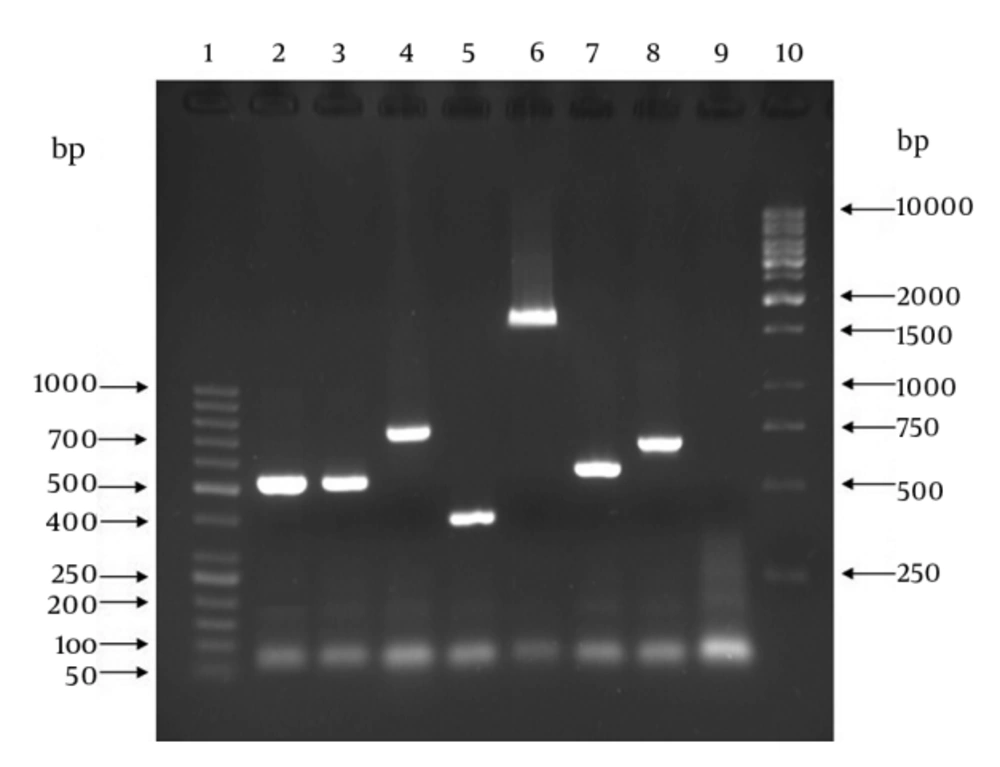
Each confirmed isolate as GBS was examined by genotyping (molecular serotyping) using multiplex PCR assays targeting nine cps genes introduced by Poyart et al. (15). For this purpose, two reaction mixes were prepared. The mix (i) contained primers for Ia, Ib, II, IV and V and the mix (ii) contained the primers for III, VI, VII and VIII. The first reaction mix contained 2.5 μL of bacterial DNA, 1 μL of each forward primer, 1 μL of each reverse primer and 12.5 μL of 2x Taq Premix-Master mix (Parstous Biotech Co, Iran). The second reaction mix contained 2.5 μL of bacterial DNA, 1 μL of each forward primer, 1 μL of each reverse primer, 12.5 μL of 2x Taq Premix-Master mix and 2 μL of sterile double distilled water. Final volume of each reaction mix was 25 μL. Amplification thermal cycles were as follows: an initial denaturation step for three minutes at 94°C followed by 30 cycles at 94°C for 30 seconds, 58°C for one minute, and 72°C for one minute and a final extension cycle at 72°C for five minutes. As with the mentioned confirmatory PCR assay methodology, PCR products were run on agarose gel and then visualized and photographed using UV transilluminator. The 50-bp and 1-kb DNA size markers (Fermentase Co, USA) were used in these assays.
3.5. Statistical Analysis
Correlation between genotypes and age groups (≤ 30 and > 30) as well as between genotypes and samples type were analyzed by Comparing Means test (independent samples T test). The test of significance was two-tailed and a P < 0.05 was considered statistically significant. All analyses were performed using SPSS version 20 software.
4. Results
In case of confirmatory PCR assay, the samples (PCR product) presenting an amplicon size of 780-bp were considered positive for GBS (Figure 1). All the 62 presumptive identified isolates produced amplicon size of 780-bp and thus were confirmed as GBS strains. Results of genotyping using multiplex PCR assay as well as correlation between samples type and genotypes are presented in Table 1. All 62 GBS strains produced amplicons from CPS related primer pairs with genotypes of Ia, Ib and II-V (Figure 2). No amplicon size indicating genotypes of VI-VIII was observed for all examined GBS strains. There were no significant correlations between age groups and genotypes (P = 0.963) (data not shown).
| Samples Type | Frequency (%) | P Valuea | |||
|---|---|---|---|---|---|
| Vaginal Swab | Urine | Blood | |||
| Genotypes | |||||
| Ia | 4 | 1 | 0 | 5 (8.1) | 0.005 |
| Ib | 0 | 2 | 0 | 2 (3.2) | 0.462 |
| II | 3 | 4 | 0 | 7 (11.3) | 0.266 |
| III | 4 | 30 | 1 | 35 (56.5) | 0.003 |
| IV | 1 | 1 | 0 | 2 (3.2) | 0.433 |
| V | 4 | 7 | 0 | 11 (17.7) | 0.346 |
| VI | 0 | 0 | 0 | 0 | - |
| VII | 0 | 0 | 0 | 0 | - |
| VIII | 0 | 0 | 0 | 0 | - |
| Total | 16 | 45 | 1 | 62 (100) | |
aP value for correlation between genotypes and samples type based on independent samples T. Test.
Lanes 1: 50-bp DNA size marker; lane 2 and 3: S. agalactiae ATCC 12386 and clinical GBS sample, respectively, with amplicon size of 521-bp denoting type Ia; lane 4: clinical GBS sample with amplicon size of 770-bp denoting type Ib; lane 5: clinical GBS sample with amplicon size of 397-bp denoting type II; lane 6: clinical GBS sample with amplicon size of 1826-bp denoting type III; lane 7: clinical GBS sample with amplicon size of 578-bp denoting type IV; lane 8: clinical GBS sample with amplicon size of 701-bp denoting type V; lane 9: sample without DNA template (as negative control); lane 10: 1-kb DNA size marker.
5. Discussion
The GBS capsule is a significant virulence factor and antigenic determinant, therefore, it is considered as one of the main targets in the investigation for development of a vaccine against GBS infections in the future. Thereby correct capsular typing of clinical isolates is essential for predicting vaccine coverage and consequently applying sensitive and specific methods is required for achieving this purpose (18-20).
The aim of current study was to differentiate genotypes of GBS clinical isolates based on PCR assay to acquire information about distribution of GBS types in Hamedan, Iran.
Among the 62 GBS isolates examined, all capsular types except for VI, VII and VIII were found. Types III and V were the most prevalent types with the sum of 46 isolates (74.2%). Type III was the predominant type with 35 (56.5%) isolates, followed by type V (11 isolates; 17.7%), type II (seven isolates; 11.3%), type Ia (five isolates; 8.1%) and Ib and IV with similar prevalence of two isolates (3.2%).
In review of prevalence of GBS types in pregnant women universally, the results were as follows: in USA, although the majority of reported studies in the 1990s showed type V as the predominant type among colonizing isolates, yet types Ia and III were more frequent in the recent years. In Canada, types Ia, III and V were common; similarly, and in Europe, except for Greece, type III was predominant in many countries (21). Additionally, type Ib is emerging in Germany (22). Based on a recent performed study in three African countries, similar prevalence of types III and V was reported. In Asian countries, after type III as the predominant type, types Ib and V were common similarly (23), however, an unusual case has been reported in Japan where types VI and VIII were predominant in Japanese pregnant women (24). In the Middle-east, types Ia, II, III and V were more frequent (23), but in the United Arab Emirates, type IV was the most common (25).
Considering the above findings, the obtained results of the current study are consistent with universal prevalence of GBS types, with types III and V as the most frequent types in many countries. Similarly, our findings are consistent with the study performed by Ippolito et al. (23), which reported types Ia, II, III and V as the most frequent types in the Middle-east.
The following results have been reported by other studies performed in Iran. In a recent study performed in Ardabil, in 2012, Jannati et al. (26) used capsular antiserum for serotyping of isolates and showed that serotypes V (19.6%), II (12.5%) and IV (12.5%) were the most frequent serotypes followed by serotypes III (10.7%) and VI (10.7%), Ib (8.9%), Ia (7/1%), VII (5/3%) and VIII (5/3%); this is while, 7.1% of strains were non-typeable. In another recent study performed by Yasini et al. (27) in Kashan, in 2012, PCR assay was used and genotyping results were as follows: the most common types were III (32.14%), V (21.43%) and IV (14.3%), respectively, followed by Ia (10.7%), VI (10.7%), Ib (7.13%) and VII (3.6%). Types II and VIII were not identified. According to the study of Nahaei et al. (28) from Tabriz, in 2007, serotypes of S. agalactiae strains were Ia (17.6%), Ib (13.4%), II (14.2%), III (9.5%), IV (8.2%), V (19.5%) and nontypable (17.6%), identified using capsular antiserum. In another study by Rahnama et al. (29) in Tehran, in 2010, it was shown that prevalence of GBS types among 50 GBS strains using PCR assay were serotype III with 25 isolates (50%) and serotype V with eight isolates (16%) as predominant serotypes, followed by serotypes Ia and II with similar prevalence of seven isolates (14%). In this study, serotypes Ib, IV, VI, VII and VIII were not found and three strains were classified as non-typeable.
Statistical associations between some types (i.e. type Ia and III) and the source of isolation were observed in current study. There is thought to be a correlation between type Ia and vaginal colonization (P = 0.005), and also between type III and urine samples (P = 0.003); however, due to the small number of samples examined in current study, these associations may not be considered as significant. The lack of statistically significant correlation between age groups and genotypes, regardless of other related studies (8), can be attributed to small number of examined samples.
The most frequent method used for GBS typing is conventional serotyping (CS) or immunological method. However, the accuracy of the obtained results is highly dependent on several agents, in fact, serological methods due to several limitations are only moderately reliable, thus, the CS method is not efficient for typing of these strains. Also, these methods can cause misidentification of certain serotypes of isolates because of problems associated with immunological cross-reaction. Being expensive and not cost-effective (particularly, commercial serotyping kits), laborious procedures and less sensitive than molecular methods, are other limitations of the CS method. With regards to the mentioned limitations, a significant proportion of isolates was non-typeable (NT) by this method. In contrast, PCR-based typing (genotyping) methods such as molecular serotyping (MS) have several applications in bacterial typing systems and show a simple modifiable level of differentiation. Molecular Serotyping identification methods are attractive due to several advantages including being specific, their high discriminatory power, clear results, high reproducibility, simplicity and fast to perform, and great availability of equipment and required materials. Advanced molecular methods have capability of differentiating strains to many variable types (4, 15, 30).
In conclusion, the results of the current study demonstrated that type III is the predominant type in Hamedan, followed by types V, type II, type Ia, Ib and IV, respectively. Using the MS method, such as the PCR assay, as an alternative to the CS method leads to accurate, sensitive, specific, and fast typing of GBS isolates; in addition, MS results reduced misclassification of non-typeable isolates, associated with CS. Therefore, MS can be used to confirm serotyping results obtained by the CS method such as latex agglutination. The advantages of MS method allow it to analyze various populations and to examine invasive and colonizing isolates in extensive epidemiological studies and surveillance activities. In fact, MS could facilitate the proper formulation of candidate GBS vaccines.