1. Background
Crimean-Congo hemorrhagic fever (CCHF) is an acute viral disease that affects various organs in the body and is diagnosed with different symptoms such as vast ecchymosis, visceral bleeding, and impaired liver function. CCHF is caused by infection with a tick-borne virus (Nairovirus) in the family Bunyaviridae. The CCHF virus can cause a severe disease with death rates ranging between 30 and 80 percent (1, 2). Patient death occurs due to hypovolemic shock caused by severe bleeding or disseminated intravascular coagulation disorder (DIC) (1, 3). Chumakov et al. (1970) (4) first identified CCHFV in 45% sheep sera that were sent from a Tehran abattoir to Moscow; however, the first human infection was not diagnosed until 1999. The disease incidence eventually grew in several provinces of Iran and the mortality polls reached 26.5 percent (2). From June 1999 to February 2011, Sistan and Baluchestan Province, in Southeast Iran, had the highest prevalence of CCHF (5). Based on research carried out in neighboring countries, one out of five cases diagnosed with CCHF normally leads to death. Middle Eastern viruses have higher intensity compared to the African viruses. The disease incidence is much higher in Pakistan and India. In recent years, the smuggling of livestock from the Eastern borders of Iran, i.e. Afghanistan and Pakistan borders, has increased the disease incidence in the neighboring provinces in Iran (6).
The disease pathogenesis could be related to soluble mediators and immune responses that lead to the direct destruction of vascular systems and parenchymal cells of target organs (7-9). The important factors related to mortality during the first five days of disease are mainly mentioned as platelet count less than 20000/mm3, ALT ≥ 900 U/L, AST ≥ 700 U/L, PTT ≥ 60 sec, and fibrinogen count less than or equal to 110 mg/dL (10). In a study, it has been suggested the level of 1060 ng/dL ferritin serum (sensitivity of 78.9% and specificity of 87%) in order to differentiate between severe and non-severe cases based on the DIC index (11). In most studies, the coagulopathy (clotting disorders) parameters in patients with CCHF and its correlation with disease severity and mortality have been proven (11, 12). In a study conducted by Mardani et al. (13), it was shown that patients stricken by low platelets, in particular those with platelets lower than 50000/mm3, are prone to high mortality rates. Patient death occurs due to hypovolemic shock caused by severe bleeding, infection, or disseminated intravascular coagulation disorder (DIC) (9, 13). Based on the clinical pathology of the disease, one of the main parameters proposed for CCHF intensity is the DIC severity that occurs amid CCHF disease (11). Furthermore, Cevik et al. (2008) (14) have suggested that platelet counts lower than 20000 per mm3, PTT ≥ 60 sec, melena presence, and decreased conscious level are related to the mortality of the CCHF disease (12). In addition, Ergunol et al. indicated platelet counts less than 20000/mm3, ALT ≥ 900 U/L, AST ≥ 700 U/L, PTT ≥ 60 sec, and fibrinogen count less than or equal to 110 mg/dL as the mortality parameters during the first five days of disease occurrence. A few studies have evaluated the role of viral load in disease intensity.
2. Objectives
Since the disease is endemic in Sistan and Baluchestan province, Iran, a comparison study regarding viral loads in CCHF patients was conducted to determine whether or not viral load is a predictor of disease severity in CCHF patients.
3. Methods
The current study was an analytical-prospective research. All probable patients admitted to Boo-Ali hospital (Zahedan, Iran) that were positively diagnosed with CCHF using serologic and PCR methods included in the study from September 2012 to March 2014. Patients with known malignant disease or diagnosed with other hematological disorders, and those who had been given antiviral, blood, or other blood products during their admittance to hospital were excluded from the study. Patients who were not interested in participation were also excluded. The data including demographic information such as age, sex, paraclinical findings, and information regarding recent disease occurrence were collected through a designed questionnaire. The blood samples (two milliliters) were collected from patients on the first and fifth days of admission to hospital. The virus RNA was extracted from plasma using High Pure Viral RNA Kit (Roche Diagnostics). The viral load was measured using RNA as a template for RT-PCR (QIAgene OneStep SYBR GREEN qRT-PCR smart mix). The values for platelet count, PT, PTT, INR, D-DIMER, and patient’s fibrinogen were recorded in the questionnaire. Next, the patients were divided into two groups of severe and non-severe viral load based on DIC severity criteria. The DIC severity scoring was based on the International society on thrombosis and haemostasis (ISTH) (11, 15).
| Score | |
|---|---|
| Platelet count | |
| > 100000/ mm3 | 0 |
| 50000 - 100000/ mm3 | 1 |
| < 50000/ mm3 | 2 |
| D-dimer | |
| 0.5 - 1 µg/mL | 0 |
| 1 - 3 µg/mL | 2 |
| > = 3 µg/mL | 3 |
| Fibrinogen | |
| > = 100 mg/dL | 0 |
| < 100 mg/dL | 1 |
| PT rising | |
| < 3 sec | 0 |
| 3 - 6 sec | 1 |
| > 6 sec | 2 |
Descriptive statistics were used to present the data and various non-parametric tests such as Mann-Whitney U (2 samples), T-test, and ROC Curve were used to analyze the data. The significance level was set at P ≤ 0.05.
4. Results
The total number of patients with suspected CCHF were 51 from whom, two cases were diagnosed with hematologic malignancy and Rheumatic diseases and consequently were excluded from the research. In addition, 12 other patients were excluded due to lack of confirmation for CCHF disease. The mean age of the remaining 37 patients (84% male, and 16% female) was 31.1 ± 12.2 years. Disease severity was determined based on DIC scoring system. According to the results of viral load on the first day of admission, 27 patients (72%) were listed in the mild CCHF group and 10 patients (28%) in the severe CCHF group. However, the results on the fifth day of admission listed 31 (83%) in the mild CCHF group and 6 (17%) in the severe CCHF group. The monitoring of the disease showed that 92% (n = 34) of the patients healed while the remaining (8%, n = 3) died due to CCHF.
4.1. Determination of viral load on the first and fifth days of admission in CCHF patients
The mean viral load in blood test samples of 34 patients (3 blood samples excluded due to poor quality) was 1.3 × 106 copies/mL on the first day of admission. The lowest and highest viral load values on the first day of admission were 4 × 103 and 7.9 × 107 copies/mL, respectively. On the fifth day, the mean viral load was 3.7 × 105 copies/mL with the lowest and highest values of zero and 3.7 × 106 copies/mL, respectively. It should be mentioned that only 24 samples were collected on the fifth day due to dissatisfaction of patients with further sampling or patients’ early discharge.
4.2. Viral Load Determination in Mild CCHF Patients Based on DIC Score
The mean viral load obtained for mild CCHF patients was 3.2 × 105 and 1.5 × 105 copy/mL on the first and fifth days, respectively. The lowest and highest viral load values for these patients on the first day were 4 × 104 and 1.8 × 105 copy/mL, respectively. These values were zero and 4.8×105 copies/mL on the fifth day, respectively.
4.3. Viral Load Determination in Severe CCHF Patients Based on DIC Score
The mean viral load obtained for severe CCHF patients was 4.3 × 106 and 1 × 105 copies/mL on the first day and fifth day of admission, respectively. The lowest and the highest viral load values for these patients on the first day was 9.8 × 105 and 7.9 × 106 copies/mL, respectively. These values were 2.1 × 105 and 3.7 × 105 copies/mL on the fifth day, respectively.
4.4. Comparison of Viral Load in CCHF Patients With Various DIC Severities (Mild or Severe)
The Mann-Whitney statistical test showed a significant difference in viral load between two groups with various DIC severities (Table 2).
| Disease Severity | Frequency | Mean (Copies/mL) | SD | Median | P Value |
|---|---|---|---|---|---|
| The first day: Mild | 27 | 3.2 × 105 | 4.2 × 105 | 1 × 105 | 0.0001 |
| The first day: Severe | 10 | 4.3 × 106 | 2.4 × 106 | 5.4 × 106 | |
| The first day: Mild | 19 | 1.5 × 105 | 1.2 × 105 | 7.2 × 104 | 0.001 |
| The first day: Severe | 6 | 1 × 106 | 1.3 × 106 | 6 × 105 |
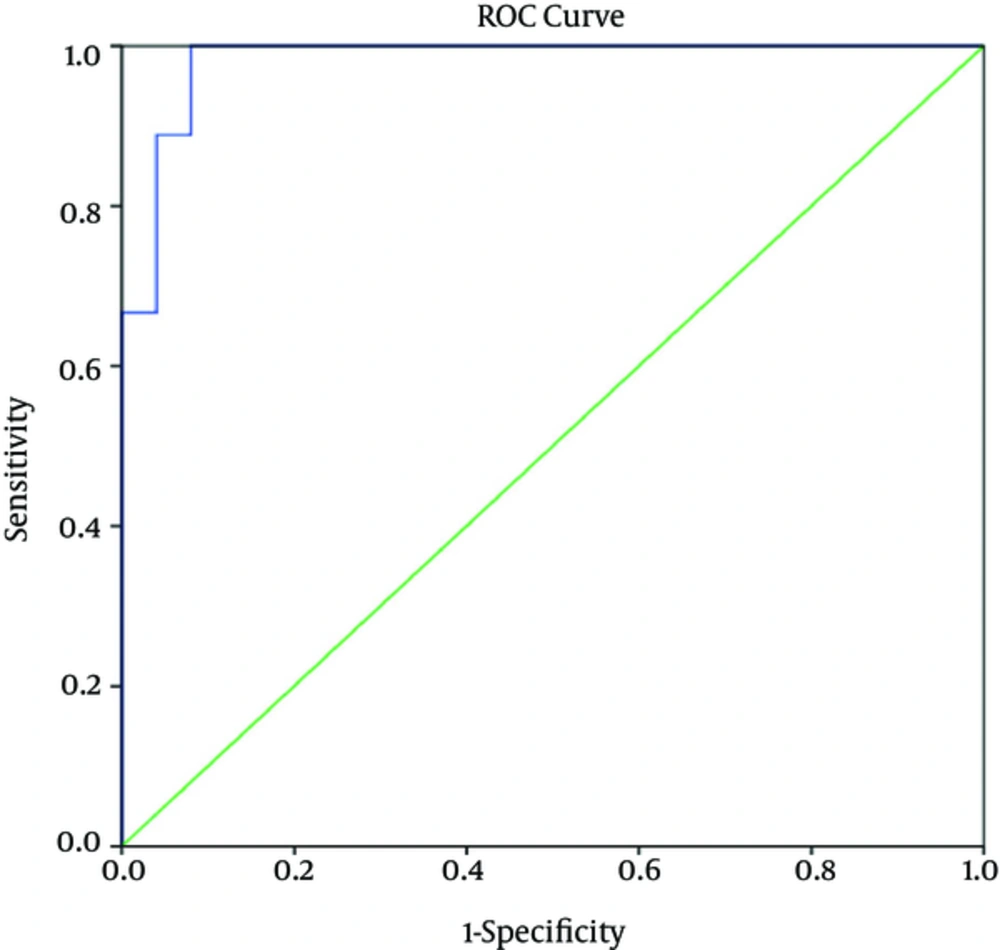
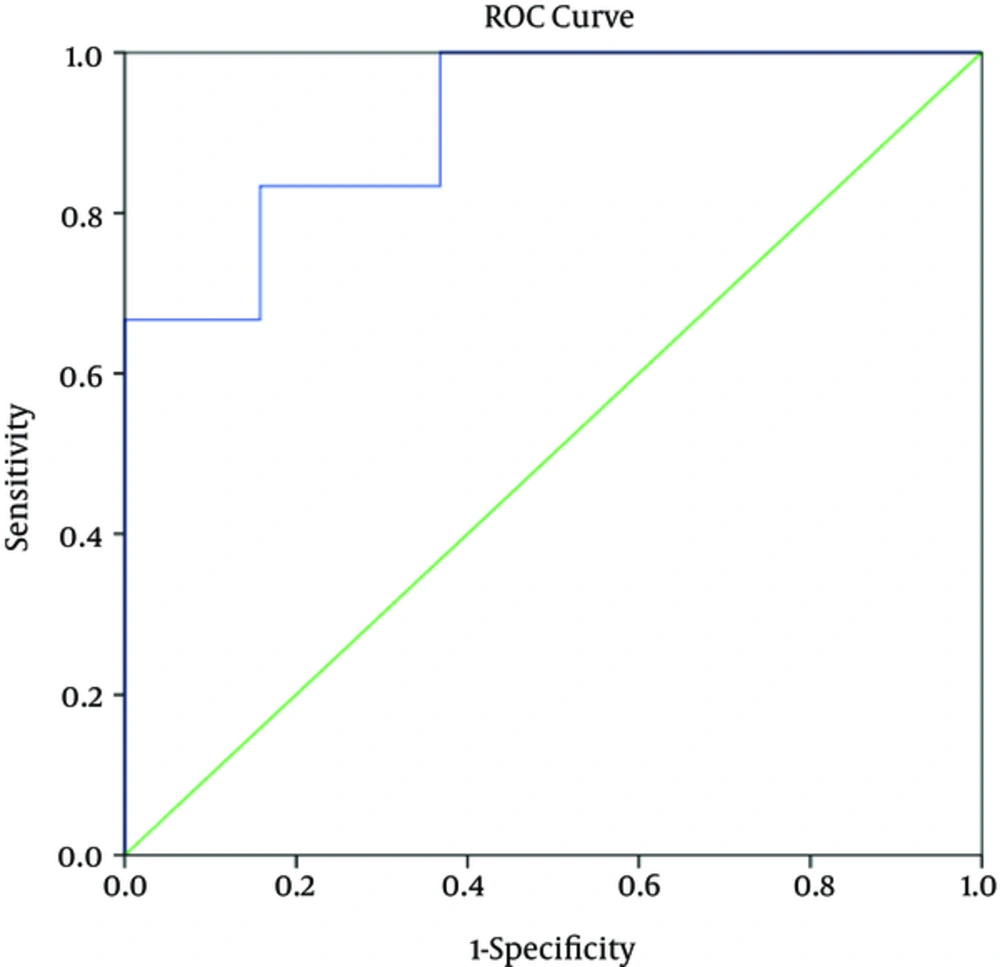
4.5. Determination of “Sensitivity”, “Specificity”, “Positive Predictive Value”, and “Negative Predictive Value” Related To Viral Load Level as Predictor of Disease Severity in CCHF Patients
Based on the ROC curves, the serum viral load level that differentiated between mild and severe cases of CCHF on the first day of admission was 8.6 × 105 copies/mL with sensitivity of 100%, specificity of 92%, positive predictive value of 82%, and negative predictive value of 100%. The corresponding values on the fifth day were 3.7 × 105 copies/mL, 83%, 84%, 62.5%, and 94.1%, respectively.
5. Discussion
In the present study, patients were divided into two groups of severe and mild CCHF based on DIC score and then, the viral load in the two groups were compared. The results indicated the mean serum viral load was 3.2 × 105 copies/mL in the mild CCHF group and 4.3×106 copies/ml in the severe CCHF group. The Mann-Whitney test showed a significant difference in serum viral load values between the groups (P ≤ 0.001). In addition, cut-off point of viral load level on the first day of admission was 8.6 × 105 copies/mL with sensitivity, specificity, positive predictive value, and negative predictive values of 100%, 92%, 82%, and 100%, respectively. The cut-off point of serum viral load on the fifth day was 3.7 × 105 copies/mL; and sensitivity, specificity, positive predictive value, and negative predictive value were 83%, 84%, 62.5%, and 94.1%, respectively. Some studies have suggested a higher viral load as an indicator for severe disease intensity. For instance, Cevik et al. (16) have indicated a viral load value higher than 1 × 109 copies/mL as predictor of a fatal outcome with positive predictive value of 80%, sensitivity of 88.9%, and specificity of 92.6%. Furthermore, the results of their study showed that the mean peak titer in patients with a fatal outcome was 7.1 × 109 copies/mL, whereas in patients with a non-fatal outcome, the mean titer was 4.1 × 106 copies/mL. In another study conducted in Kosovo (by Duh et al. (17)), patients were divided into three different groups of fatal, severe, and moderate (based on clinical and Para-clinical findings). A viral load higher than 108 copies/mL was selected to differentiate between fatal and recovered groups. The mean viral loads in the fatal and recovered groups were 1.78 × 106 and 8.06 × 106 copies/mL, respectively. As mentioned above, the comparison criteria differ from one study to another; for instance in some studies, two groups of patients including patients with recovered and fatal outcomes were compared with each other (16, 17). Up to now, various parameters have been proposed to determine disease severity including platelet counts lower than 50000/ mm3, increased levels of PTT, PT, Liver transaminases, muscle enzyme, and viral load levels. However, researchers have a common agreement that severity of DIC, which occurs during CCHF, can be used as a prediction factor for disease severity (11, 15, 18). In the current study, three cases of fatal outcome occurred with initial platelet counts of 4000, 19000, and 10000/ mm3. Their liver enzyme levels (ALT of 1612, 280 and 250; AST of 9197, 240 and 200, respectively) were other indicators of severe disease. A significant difference was observed in viral load, platelet levels, and coagulation factors such as INR and PT (P < 0.05) that were associated with the severity of the disease. A platelet count under 50000/ mm3 was described as fatal indicator by Mardani et al. Also, six different parameters including platelet, D-Dimer, INR, PT, PTT, and fibrinogen were suggested as disease severity indicators by Mehrabi et al. and Hasanoglu et al. (9, 13, 19).
The current research faced two main limitations. First, the time of admission was different in the patients. Second, some of the patients were discharged earlier or did not further consent to be kept at the hospital or cooperate with our study.
In summary, the results indicated a significant relationship between viral load and disease severity in patients with CCHF. Based on the results, it is highly suggested to measure viral load of admitted patients with suspected CCHF. This would allow admitting patients with high viral load as “high-risk” patients.