1. Background
Tuberculosis (TB) is a major public health problem in the world despite various control efforts and therapeutic strategies. Mycobacterium tuberculosis (MTB), which is resistant to several first-line TB drugs, especially rifampicin and isoniazid, and is called multi-drug resistant TB (MDR-TB), is a global health threat. The total number of global cases of MDR-TB increases annually. Indonesia was one of seven countries with the largest number of drug-resistant (DR) TB patients, based on the World Health Organization (WHO) global report in 2022 (1).
Rifampicin is known for its inhibitory effects on actively growing TB bacilli. Rifampicin works by binding a subunit from the ribonucleic acid (RNA) polymerase and avoiding RNA messenger elongation.
Rifampicin-resistant TB (RR-TB), which is detected using genotypic or phenotypic methods, can be resistant or non-resistant to other first-line anti-TB drugs. Rifampicin-resistant TB is usually used as a surrogate marker for MDR-TB because 90% of the RR-TB isolates are also resistant to isoniazid. Accordingly, RR-TB is categorized as MDR-TB and given treatment regimens as an MDR-TB (2). The national TB program in Indonesia is based on WHO recommendations. Rifampicin-susceptible cases are considered drug-susceptible (DS) TB and are given the standard regimen of DS-TB, whereas new cases of RR-TB that are tested twice and have the same results are treated as MDR-TB.
Multi-drug resistant TB is usually associated with high morbidity and mortality compared with DS-TB, but several researchers considering MTB strains that were resistant to various TB drugs experience reduced fitness due to mutations, which is called the fitness cost theory. This theory focused on decreased virulence and reduced ability of bacilli to reproduce and transmit (3). The reduced fitness reduces the destructive power of MTB for the network on the host. A comparison of infectivity between DR and DS-TB strains is still controversial (4). The development of DR-TB infections, when compared with DS-TB ones, was not well understood. Studies on guinea pigs and in vitro experiments state that there has been a decrease in the fitness level of MTB that has mutated to become DR (5).
Apart from being a diagnostic tool, chest X-rays can provide information on the degree of lung damage in TB (6). Assessment with a numerical scoring system could describe the severity of pulmonary TB by assessing chest X-ray images (7). The lesion severity score of lung damage in pulmonary TB patients is obtained by assessing the area of the lesion and cavity (8). Chest images are preferred over chest CT because they are available in almost all primary health facilities and still show the morphology of the lesion, such as cavities, consolidation, pleural effusion, and fibrosis (9).
2. Methods
This was an analytical observational study with a retrospective cross-sectional design.
The subjects were new cases of pulmonary TB based on sputum Xpert MTB/RIF examination at Dr. Soetomo General Hospital (Surabaya, Indonesia), where the sampling was performed from January 2018 to November 2022. A new case is a new patient who has not received or has received anti-TB drugs in less than one month. The results of MTB detection susceptible to rifampicin were sufficient based on one examination and declared the case as DS-TB, while the results for MTB detection with rifampicin resistance based on two examinations were expressed as DR-TB. The inclusion criteria were adults (> 18 years old) with medical records, including chest X-rays, before undergoing treatment. The exclusion criteria were pulmonary TB patients with a history of TB treatment for one month or more and patients with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS).
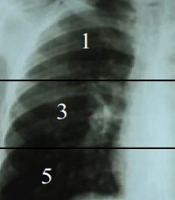
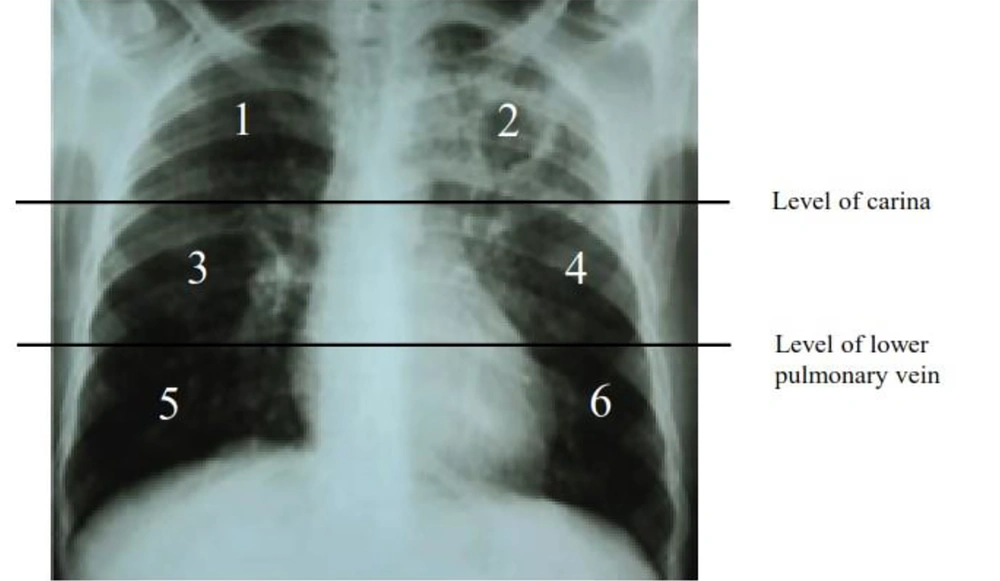
The data collected were secondary data consisting of medical records, such as demographical information, sputum Xpert MTB/RIF result, and chest radiography. Chest X-ray data was carried out by the same expert radiologist who was included in this research team in the thoracic division and a pulmonary specialist in the infection division who was experienced in TB cases and was blinded as to the drug resistance results which would be assessed for the degree of lung damage using the severity scoring on chest X-ray. On the chest X-ray, a horizontal line was drawn through the tracheal bifurcation, delimiting the upper and middle lung fields. Similarly, a horizontal line was drawn through the lower pulmonary vein, separating the middle and lower pulmonary fields. In total, the two lung fields were divided into 6 zones, and each zone was denoted as 1 to 6 (Figure 1).
There were 3 types of chest X-ray severity score criteria based on the area of the lesion.
First, lung damage with no lesion was marked by 0, a lesion area < 50% was marked by 1, and a lesion area > 50% was marked by 2. Moreover, there were 7 types of chest X-ray severity score criteria based on cavity characteristics. No cavity was marked by 0, a single cavity < 2 cm in diameter was marked by 0.25, a single cavity 2 - 4 cm in diameter was marked by 0.5, and a single cavity > 4 cm in diameter was marked by 1. Multiple cavities with the largest diameter < 2 cm were marked by 0.5; multiple cavities with the largest diameter of 2 - 4 cm were marked by 1; and multiple cavities with the largest diameter > 4 cm were marked by 2.
Lung damage severity belonged to the mild category if it had a total score < 2.5, the moderate category if it had a total score of 2.5 - 6, and the severe category if it had a total score > 6 (8). In this study, the severity rate was modified to mild-moderate (≤ 2.5 - 6 score) and severe (> 6 score).
Statistical processing and analysis were performed in IBM SPSS statistic version 21.0 (IBM, Chicago, IL, USA) to compare differences in lung damage on chest X-rays between new cases of DR and DS pulmonary TB. A P-value of 0.05 or less was regarded as significant. The research protocol was approved by the Ethics Committee of Dr. Soetomo General Academic Hospital, with the exemption letter number 1039/LOE.301.4.2/IX/2022.
3. Results
A total of 319 patients, consisting of 132 new cases of DR pulmonary TB and 187 new cases of DS pulmonary TB were recruited. Their mean age was 43.64 ± 15.56 years, with an age range of 18 - 85 years and a median age of 45 years. Moreover, the majority of the patients (n = 179, 59.5%) were male. There were 85 subjects (28.2%) who had comorbidities, including diabetes mellitus (DM), with a percentage of 21.9% in DR pulmonary TB and 23% in DS pulmonary TB. The characteristics of the subjects are presented in Table 1.
| Variables | DR Pulmonary TB (N = 132) | DS Pulmonary TB (N = 187) | P |
|---|---|---|---|
| Age (y) | 0.249 | ||
| Range | 18 - 73 | 18 - 85 | |
| Median | 45.0 | 45.0 | |
| Mean ± SD | 42.53 ± 14.52 | 44.47 ± 16.03 | |
| Sex | 0.506 | ||
| Male | 70 (53.1) | 119 (63.6) | |
| Female | 62 (46.9) | 68 (36.4) | |
| Comorbidity | 0.000 | ||
| Diabetes mellitus | 29 (21.9) | 43 (23.4) | |
| Hypertension | 0 (0) | 4 (2.1) | |
| Hepatitis | 0 (0) | 3 (1.6) | |
| Chronic kidney disease | 0 (0) | 3 (1.6) | |
| Chronic obstructive pulmonary disease | 0 (0) | 3 (1.6) | |
| Asthma | 1 (0.7) | 2 (1.1) | |
| Others | 0 (0) | 2 (1.1) |
a Values are expressed as No. (%) unless otherwise indicated.
Drug-resistance pulmonary TB and DS pulmonary TB patient had almost ages, respectively 44.47 ± 16.03 and 42.53 ± 14.52 years. There was no significant difference in the age variable (P = 0.249). The frequency of men in the DR pulmonary TB group was higher (n = 70, 53.1%) compared to that of women (n = 61, 46.9%). The same was true for the DS pulmonary TB group, in which men (n = 119, 63.6%) were more than women (n = 68, 36.4%). The sex variable was not significant (P = 0.506).
There were 29 subjects (21.9%) with comorbid DM in the DR pulmonary TB and 43 subjects (23.4%) in the DS pulmonary TB, followed by hypertension (2.1%), hepatitis (1.6%), chronic kidney disease (CKD) (1.6%), chronic obstructive pulmonary disease (COPD) (1.6%), and other diseases (1.1%) which were only found in DS pulmonary TB. Comorbid asthma was found in 1 patient (0.7%) in DR pulmonary TB and 2 (1.1%) in DS pulmonary TB. The comorbid variable was significantly different (P = 0.000).
The level of lung damage on chest X-ray was assessed according to the modified severity criteria, with two levels of severity: Mild-moderate and severe (9).
Among the new cases of DS pulmonary TB, the severity rate on chest X-rays was dominated by the severe category (Table 2).
| Severity Degree | DR Pulmonary TB No. (%) |
|---|---|
| Mild-moderate | 68 (51.5) |
| Severe | 64 (48.5) |
| Total | 132 (100) |
Abbreviations: DR, drug-resistant; TB, tuberculosis.
The frequency of lung damage levels on the chest X-ray of new cases of DS pulmonary TB is shown in Table 3.
| Severity Degree | DS Pulmonary TB No. (%) |
|---|---|
| Mild-moderate | 43 (23) |
| Severe | 144 (77) |
| Total | 187 (100) |
Abbreviations: DS, drug-susceptible; TB, tuberculosis.
Among the new cases of the DR pulmonary TB group, the severity rate on chest X-rays was dominated by the mild-moderate category (Table 4).
| Severity Degree | DR Pulmonary TB, No. (%) | DS Pulmonary TB, No. (%) | P |
|---|---|---|---|
| Mild-moderate | 68 (51.5) | 43 (23) | < 0.001 |
| Severe | 64 (48.5) | 144 (77) | |
| Total | 132 (100) | 187 (100) |
Abbreviations: DS, drug-susceptible; DR, drug-resistant; TB, tuberculosis.
There were more subjects with lung damage on chest X-ray in the severe category than in the mild-moderate category among the new cases of DS pulmonary TB (77% vs. 23%).
Instead, in the DR pulmonary TB group, there were more subjects with lung damage on chest X-ray in the mild-moderate category than in the severe category (51.5% vs. 48.5%). There was a significant difference in lung damage on chest X-ray between new cases of DS pulmonary TB and DR pulmonary TB (P < 0.001).
4. Discussion
The age range of the subjects was 18 - 85 years, with an average age of 42.53 years among the DR pulmonary TB patients and 44.47 years among the DS pulmonary TB patients. Another study stated the average age to be 33 years in new cases of DR pulmonary TB and 30 years in DS pulmonary TB (10). Other studies also stated that the average age of DR pulmonary TB cases was 34 years (11). The average age of DR and DS pulmonary TB in this study was higher compared to other similar studies. The number of men was higher in this study compared to women. This study was also consistent with the global report in 2022 conducted in 15 Asian countries, showing that > 50% of men were infected with TB compared to women (1).
Comorbid DM was found to be the highest in both DR and DS pulmonary TB, followed by hypertension, hepatitis, CKD, COPD, asthma, and other diseases. A systematic review of 13 observational studies found that DM increased the risk of TB by up to three times. Of the 10 countries with the highest number of DM patients in the world, six countries, including Indonesia, were classified by WHO as having a high burden for the occurrence of TB with DM (12). The incidence of pulmonary TB with DM is caused by the failure of the defense system. In this case, the lungs experience impaired function of the respiratory epithelium and ciliary motility. Impaired function of the pulmonary vascular capillary, rigidity of the red blood cell corpus, and changes in the oxygen dissociation curve due to prolonged hyperglycemia were important factors in the failure of the defense mechanism against infection.
The spread of MTB from the lung parenchyma into the airways and cavity formation requires the destruction of the extracellular matrix (ECM). Only the matrix metalloproteinases (MMPs) can completely degrade ECM, so MMPs play an important role in the development of cavities, bronchiectasis of cavities, and fibrosis in pulmonary TB (13, 14). Increased levels of MMPs affect lung damage; sputum levels of MMP-1, MMP-2, and MMP-8 were increased in patients with cavities and were positively correlated with the degree of damage on chest X-ray.
The level of lung damage on the chest X-ray of new cases of DR pulmonary TB group was dominated by the mild-moderate category, while in the DS pulmonary TB group, the level of lung damage was dominated by severe category. This finding was consistent with the fitness cost theory, which states that DR pulmonary TB strains experience a decrease in fitness compared to DS strains. This was based on the perception that mutations occurring in DR strains adversely affect the normal physiological function of pathogens. Drug-resistant mutants were expected to grow slowly or exhibit less virulence. Drug target gene mutations in MTB conferred drug resistance at the expense of the physiological fitness of the pathogens, thereby making them less able to proliferate even in nutrient-rich environments (15). It was even proven in theory that the fitness cost did not mean that the MTB in MDR TB could be ignored and not treated well because although these bacteria are weak, they are difficult to die. This is in line with previous research, which stated that it could be a sneak attack developed during latent TB as an asymptomatic obstructive lobular pneumonia (16).
The severity of lung damage was not only affected by TB virulence. This severity could be broadly influenced both epidemiologically and by host and environmental conditions. Host conditions such as the presence of immune granulomas produced in the lungs could affect the growth of MTB. In addition, the prevalence of individuals living in slums and poor areas could increase infection and exacerbate pulmonary TB. This situation indirectly affects the nutritional conditions of both macro- and micronutrients. Vitamin D deficiency has also been known to affect the immune system; it can increase respiratory diseases, including the severity of pulmonary TB (17, 18). Clinically, pulmonary TB patients with unmet weight loss and malnutrition can be caused by inadequate intake or consequences of TB, such as anorexia, metabolic dysfunction, and poor absorption (19). These epidemiological and clinical factors directly influence the severity of lung damage in pulmonary TB patients.
Genetics was the factor that may have affected the severity of lung damage in pulmonary TB patients. Research shows that single nucleotide polymorphisms (SNPs) on the toll-like receptor (TLR)-1, TLR-2, and TLR-6 of Indonesian MDR pulmonary TB patients were found to have a relationship with disease severity. TLR polymorphisms had a significant relationship with TLR-1, TLR-2, and TLR-6. TLR gene polymorphisms could explain various natural immune responses to MTB, which cause different disease manifestations of the severity of TB (20).
The limitation of this study was not analyzing the factors such as epidemiological, clinical, and genetic factors related to lung damage. This study examined whether there was resistance based on the results of the Xpert MTB/RIF examination, which only showed the presence or absence of resistance to rifampicin with the assumption that recording to the national program which cases with Rifampicin sensitivity were treated as DS pulmonary TB while cases with Rifampicin resistance were managed as MDR-TB patients. Patients with other forms of resistance, such as isoniazid, pyrazinamide, and ethambutol, were not included among the new cases of DS and DR pulmonary TB. Besides, the lung damage severity analysis was not conducted by thoracic computerized tomography (CT) scan thorax, which is more sensitive and specific than X-ray images; because the study was retrospective, it only used the X-ray images as a diagnosis from daily practice.
4.1. Conclusions
The severity of DR pulmonary TB was mostly in the mild-moderate category, while in DS pulmonary TB, it was mostly in the severe category. There was a difference in lung damage on chest X-ray between new cases of DR and DS pulmonary TB. Future studies can examine factors that influence the severity of lung damage more broadly, including the host and environmental factors, and perform severity assessments based on chest CT scans.