1. Background
Toxoplasma gondii is a protozoan member of the phylum Apicomplexa, which can cause severe infection in warm-blooded animals, such as humans and animals (1). Considering the global infection and spread of this parasite, it is recognized as one of the largest public health problems in one-third of the world’s population. It is mostly transmitted through consumption of undercooked or raw meat, containing tissue cysts or oocysts excreted from the definitive host (2, 3). Sporozoites can influence the digestive tract and are released when reaching the bloodstream (4). T. gondii can live and reproduce in a variety of host tissues, such as the heart, liver, kidneys, skeletal muscles, eyes, central nervous system, and immune system cells (1, 5).
Toxoplasmosis is a self-limited disease in people with a healthy immune system. However, in people with immunodeficiency, severe clinical symptoms may appear, leading to the death of patients in some cases (6, 7). T. gondii parasite can infect most tissues, although clinical symptoms are variable, depending on the site of infection. Therefore, severity of symptoms and pathological lesions is associated with congestion and tachyzoites in different tissues (8).
Most studies on parasite burden have focused on mouse models of toxoplasmosis, using microscopic observation of tissue samples. This method has disadvantages, such as low sensitivity, time-consuming design, and possibility of error (9, 10). Different polymerase chain reaction (PCR) and real-time PCR (quantitative PCR or qPCR) assays have been developed for research on T. gondii (11). The most important disadvantage of these methods is that they are time-consuming and do not provide quantitative data.
The advent of qPCR technique has been shown to be useful for different purposes, including pathogen identification, gene expression, and quantitative detection (12). Overall, qPCR is a very sensitive, accurate, and rapid technique for DNA quantification (12, 13). This technique has been previously employed with SYBR green or TaqMan probes for identification and quantification of T. gondii parasites in various types of materials (14).
Infection with T. gondii is one of the problems in tissue transplantation; therefore, identification of tissues with a higher possibility of parasite load can be helpful. In people with immunodeficiency, including AIDS patients, determination of the parasite load in tissues can be useful in treatment and disease control strategies. With this background in mind, the present study was designed to evaluate the exact orientation and burden of T. gondii in various tissues of mice infected with toxoplasmosis via qPCR.
2. Methods
2.1. Parasites
Tachyzoites of RH strain were intraperitoneally injected in mice. Seventy-two hours after infection, tachyzoites were aspirated from the peritoneal cavity (15), and the parasite suspension was washed twice with sterile phosphate-buffered saline (PBS; pH, 7.4), containing 100 IU/mL of penicillin and 100 µg/mL of streptomycin. The number of parasites was identified as 1 × 104/mL via counting by a hemocytometer under the microscope light (× 400 magnification) (16).
2.2. Laboratory Mice
In this study, male and female BALB/c mice (age, 4 - 6 weeks; weight, 23 - 18 g) were purchased from Zahedan Animal House (Sistan and Baluchestan, Iran). The project was approved by the ethics committee of Zahedan University of Medical Sciences.
2.3. Experimental Design
Male and female mice were divided into 2 groups, and 1 × 103 parasites/mL of RH-strain tachyzoites was intraperitoneally injected in mice. Following that, the male and female groups were randomly divided into 6 groups (n, 7). PBS was injected in the negative healthy control group (n, 5), and the animals were sacrificed at different intervals. The assessments were performed at 1, 2, 3, 4, and 5 days postinoculation (dpi), as well as the time of death; the mice were monitored 3 times a day. Afterwards, blood and other organs, including the heart, brain, liver, lung, genitals, spleen, eyes, kidneys, and muscles, were harvested, weighed separately, washed in saline, and stored at 20°C to extract DNA.
2.4. PCR Experiments
For tissue DNA extraction, approximately 25 - 50 mg of disrupted organ segments was homogenized, and then, 200 of tissue lysis buffer was added. Afterwards, blood and tissue DNA was extracted using DynaBio Blood/Tissue DNA Extraction Mini Kit (Cat. No., L-94044) according to the manufacturer’s instructions. Moreover, the 98-bp repetitive element of T. gondii B1 gene (Genbank, AF179871.1) was used as the target gene.
Amplification was performed using the qPCR assay. Each amplification included a positive control (total DNA extracted directly from RH-strain tachyzoites) and a negative control (distilled water or DNA extracts from a blood sample of a T. gondii-negative mouse). Briefly, 660 ng (5 µL) of template DNA was added to a reaction mixture, containing 12.5 µL of 2X qPCR Master Mix Green-High Rox (Ampliqon, Denmark), 160 pmol (2 µL) of forward primer TOXO F (2 µL; 5´-TCCCCTCTGCTGGCGAAAAGT-3´), 160 pmol (2 µL) of reverse primer TOXO R (2 µL; 5´-AGCGTTCGTGGTCAACTATCGATTG-3´), and 3.5 µL of DEPC-distilled water in a final volume of 25 µL.
The qPCR assay was performed with the StepOne™ Real-Time PCR system (Applied Biosystems). The reactions were performed at 95°C for 10 minutes, at 95°C for 20 seconds (40 cycles of denaturation), at 58.5°C for 30 seconds (annealing), and at 72°C for 30 seconds (amplification) (12). The SYBR green assay was used to optimize the annealing temperature of the primer pair, using a temperature gradient. The melting curve analysis verified the correct product size and did not result in the formation of side products or primer dimers. Samples were run in triplicate, and the means were calculated.
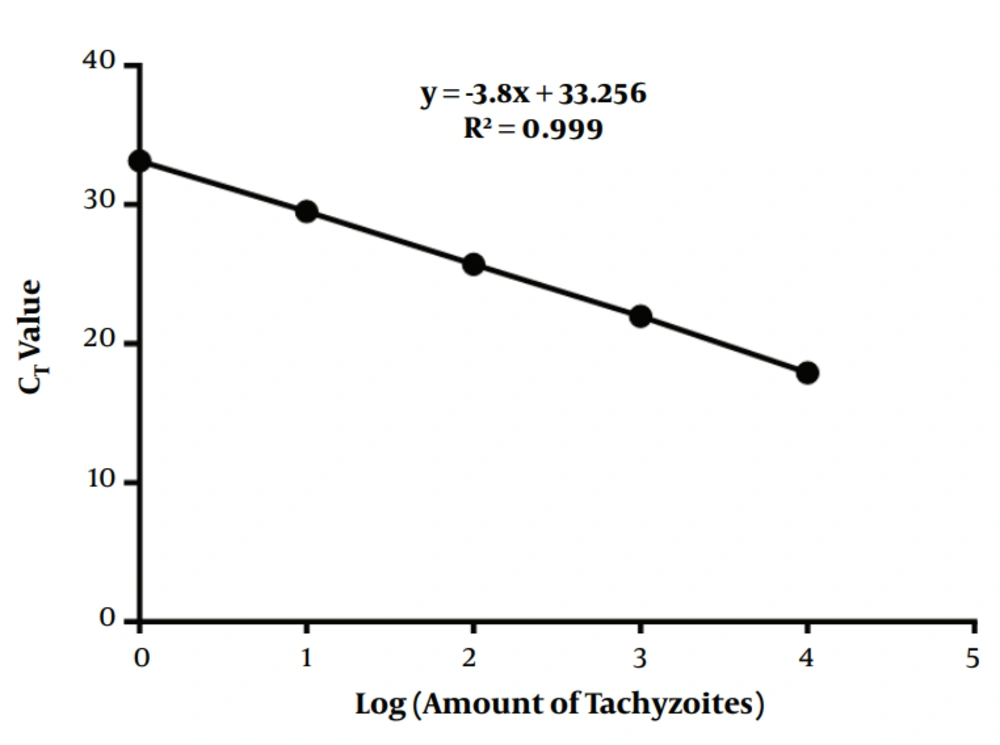
The number of parasites in the samples was calculated using the qPCR threshold cycle (CT), according to the standard curve (linear curve slope, -3.8; Y intercept, 33.254; R2, 0.999) from DNA samples in 10-fold serial dilutions (parasites/mL) of RH-strain tachyzoites (Figure 1 and Table 1). The results are expressed as T. gondii tachyzoite equivalents per µL of blood and per mg of tissue.
| Tachyzoites | CT |
|---|---|
| 10000 | 17.91 |
| 1000 | 21.98 |
| 100 | 25.73 |
| 10 | 29.52 |
| 1 | 33.14 |
2.5. Statistical Analysis
Kruskal-Wallis test was used to compare DNA quantification among tissues at different intervals at a significance level of 0.05. Furthermore, Mann-Whitney U test was performed for multiple comparisons of means between every 2 tissues and every 2 intervals through Bonferroni correction at significance levels of 0.006 and 0.012, respectively.
3. Results
Following the intraperitoneal injection of T. gondii strains, all mice died within 4 - 5 days after infection. The qPCR analysis was conducted on samples collected at different intervals to determine the exact amount of T. gondii DNA in tissues and blood of infected mice. Infection of BALB/c mice with T. gondii RH strain was easily measurable via qPCR assay at 1 day postinoculation (Figure 2). The samples were tested twice, and no T. gondii DNA was observed in the negative control wells (uninfected mice).
3.1. Parasite Burden in Samples from Female Mice
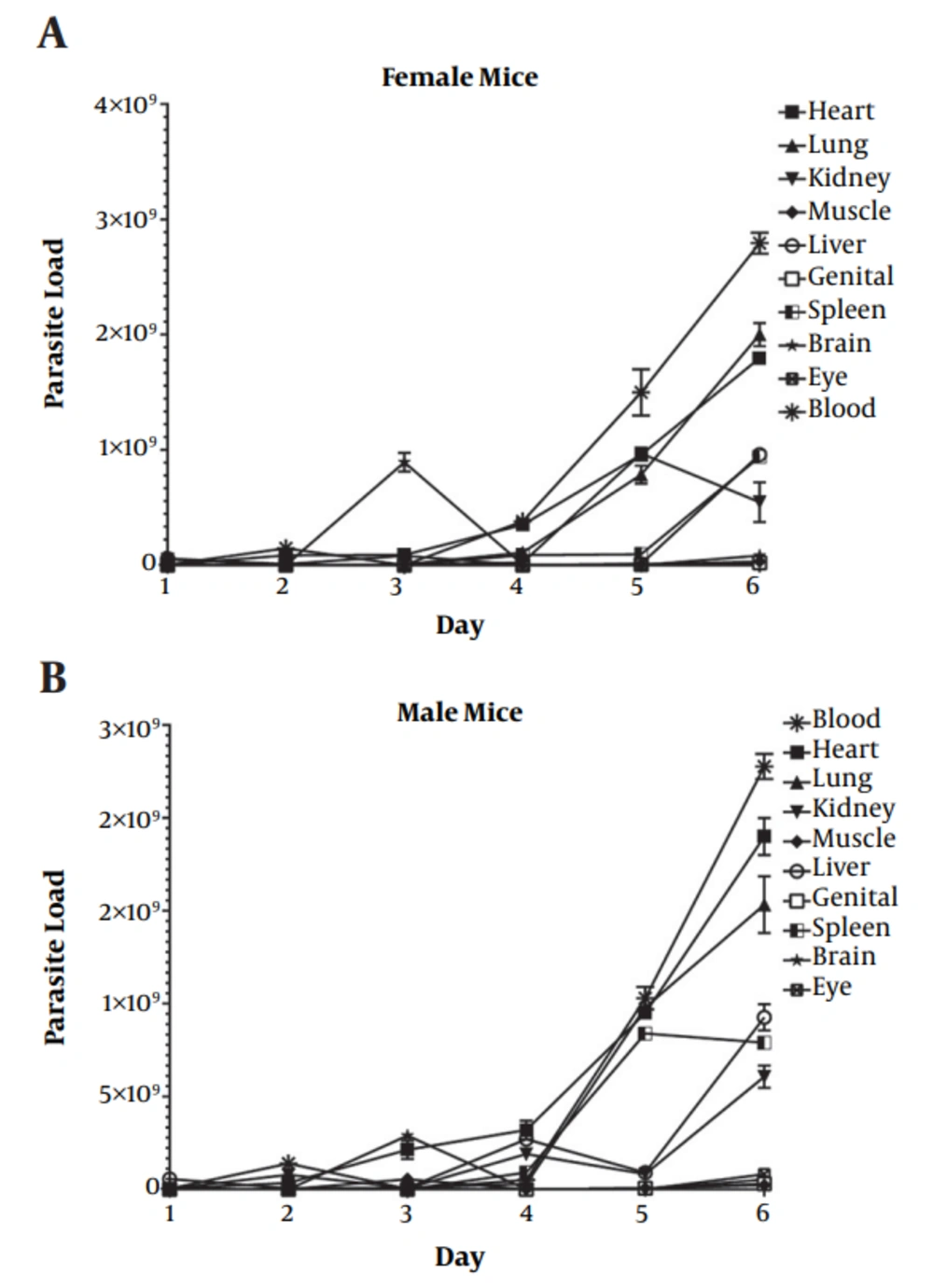
On the first day after infection, the highest parasite burden was observed in the liver and then kidneys, while the minimum copy number was observed in the eye, brain, and muscle tissues. On the second day after inoculation, the highest amount of T. gondii DNA was reported in the heart and blood, while the parasite burden was the lowest in the genitals. On the third day after intraperitoneal inoculation, the highest parasite load was detected in the brain, while low parasite count was found in the spleen. On the fourth day, the parasite load increased in the blood, heart tissues, and then the lungs. Finally, on the fifth day and at the time of death, the highest parasite burdens were detected in the blood and tissues of the heart, liver, and kidneys, respectively (Figure 3).
3.2. Parasite Burden in Samples from Male Mice
On the first day after infection in male mice, the highest parasite burden was observed in the liver, while the minimum copy number was reported in the lung and brain tissues. On the second day after parasite inoculation, the highest quantity of T. gondii was observed in the blood and kidney tissues, while the parasite burden was the lowest in the muscles. On the third day after the injection of tachyzoites, the highest parasite load was detected in the brain and heart, while low parasite levels were found in the spleen and eyes. On the fourth day, an increase was observed in the parasite burden of the heart, liver, and kidney tissues. Finally, on the final day (fifth day) and at the time of death, the highest parasite burdens were detected in the blood and lung and heart tissues, respectively (Figure 3).
The parasite kinetics and parasite burden in various tissues showed significant differences between male and female groups. However, the highest parasite burden in male and female mice was observed in the blood and heart tissues at the time of death (Table 2).
| Tissues | Groups and P Value | Days Postinfection (Parasite Load) | |||||
|---|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | Fifth | Time of Death | ||
| Blood | Male | 4.1 × 106 | 1.4 × 108 | 3.3 × 106 | 5.2 × 107 | 1.0 × 109 | 2.3 × 109 |
| Female | 4.5 × 106 | 1.5 × 108 | 9.9 × 105 | 3.8 × 108 | 1.7 × 109 | 2.8 × 109 | |
| Heart | Male | 4.4 × 106 | 3.5 × 107 | 2.2 × 108 | 3.2 × 108 | 9.5 × 108 | 1.9 × 109 |
| Female | 5.1 × 106 | 8.6 × 107 | 8.9 × 107 | 3.5 × 108 | 9.6 × 108 | 1.8 × 109 | |
| Lung | Male | 7.9 × 103 | 5.1 × 105 | 9.8 × 106 | 2.9 × 107 | 9.8 × 108 | 1.5 × 109 |
| Female | 5.3 × 104 | 3.9 × 105 | 8.2 × 106 | 1.1 × 108 | 8.1 × 108 | 2.0 × 109 | |
| Kidney | Male | 4.2 × 106 | 7.9 × 107 | 6.9 × 106 | 1.9 × 108 | 8.3 × 107 | 6.2 × 108 |
| Female | 5.1 × 107 | 6.8 × 106 | 7.3 × 106 | 2.3 × 107 | 9.7 × 108 | 7.4 × 108 | |
| Muscle | Male | 1.2 × 104 | 2.4 × 103 | 5.5 × 107 | 5.7 × 105 | 6.4 × 106 | 2.4 × 107 |
| Female | 4.2 × 103 | 5.2 × 104 | 5.9 × 106 | 8.9 × 104 | 8.4 × 106 | 3.2 × 107 | |
| Liver | Male | 5.6 × 107 | 6.3 × 106 | 8.7 × 106 | 2.7 × 108 | 9.1 × 107 | 9.4 × 108 |
| Female | 5.9 × 107 | 9.3 × 106 | 8.2 × 107 | 3.1 × 106 | 1.2 × 107 | 9.6 × 108 | |
| Genitals | Male | 1.5 × 104 | 2.7 × 104 | 4.7 × 105 | 6.1 × 104 | 6.3 × 106 | 3.1 × 107 |
| Female | 2.1 × 104 | 1.8 × 104 | 5.1 × 105 | 8.1 × 105 | 6.7 × 106 | 2.2 × 107 | |
| Spleen | Male | 1.4 × 104 | 4.3 × 105 | 2.1 × 105 | 9.1 × 107 | 8.4 × 108 | 7.9 × 108 |
| Female | 1.6 × 104 | 2.1 × 106 | 2.3 × 105 | 8.8 × 107 | 9.5 × 107 | 9.4 × 108 | |
| Brain | Male | 8.5 × 103 | 7.3 × 105 | 2.9 × 108 | 9.8 × 105 | 7.3 × 105 | 5.4 × 107 |
| Female | 9.2 × 103 | 7.7 × 105 | 9.3 × 108 | 7.0 × 106 | 8.1 × 104 | 8.5 × 107 | |
| Eyes | Male | 8.9 × 103 | 3.0 × 104 | 1.2 × 105 | 9.1 × 104 | 1.1 × 106 | 8.2 × 107 |
| Female | 9.2 × 103 | 7.2 × 104 | 2.6 × 105 | 4.8 × 105 | 5.7 × 105 | 1.5 × 107 | |
Kruskal-Wallis test was used to compare DNA quantification among tissues at different intervals at a significance level of 0.05. Mann-Whitney U test was performed for multiple comparisons of means between every 2 tissues or every 2 intervals through Bonferroni correction at the significance levels of 0.006 and 0.012, respectively.
4. Discussion
In this study, male and female BALB/c mice were infected with 1 × 103 parasites/mL of tachyzoites from T. gondii RH strain. Using the qPCR assay and T. gondii B1 gene targeting, the exact parasite count was investigated per mL of infected blood and per g of brain, liver, kidney, spleen, heart, muscle, genitals, and eye tissues. The parasite burden was evaluated on different days within 24 hours after infection on a daily basis until death. All tissues collected from the infected mice were positive for the parasites during the study. The highest parasite burden was detected at death time in the blood and heart tissues of both groups. On the other hand, the lowest parasite burden was observed in the brain and eye tissues at the time of death.
The kinetics and distribution of T. gondii have been previously examined using techniques with low sensitivity and high error rates. These methods cannot accurately measure the amount of parasites per g of tissue or per mL of blood. However, with advances in laboratory techniques and available facilities, it is possible to examine the kinetics and count of T. gondii in tissues. The kinetics of T. gondii has been studied in previous studies, and the parasite burden has been determined in different tissues to evaluate the severity of infection and effects of antiparasitic drugs and vaccines; also, diagnosis of ocular toxoplasmosis has been proposed (17-19).
In the current study, considering the low sensitivity and long lead time of staining techniques, the qPCR assay, which is a very sensitive technique for detecting and quantifying parasite burden in tissues and blood, was applied, and 0.005 of parasites was used per reaction (12, 16, 20). In this regard, in a study by Aigner et al. on Toxoplasma load in the brain and heart tissues of chickens with positive serological tests (via qPCR), no significant difference was reported in the quantity of parasites between the tissues (21). Moreover, Jurankova et al. evaluated the quantity of Toxoplasma in goat tissues, including the brain, lung, spleen, liver, heart, and kidney tissues, using qPCR. The highest parasite burden was detected in the lungs, followed by infected brain tissues; however, low levels of infection were observed in other tissues, and heart and kidneys showed the lowest level of parasites (20).
Djurkovic et al. examined the kinetics and distribution of T. gondii in the brain, liver, lung, and blood tissues, and an infected mouse model was analyzed via qPCR technique, tracking B1 gene. All samples were infected a day after intraperitoneal inoculation (24 hours postinfection) with 1 × 106 parasites/mL of tachyzoites of Toxoplasma RH strain. The infection was positive, and the samples showed the highest parasite burden in the blood and the lowest DNA copy number in the brain (15). Moreover, in a study by Dadimoghaddam et al. on tissue tropism and parasite burden of T. gondii in mice, the highest parasite burden was reported in the kidney, heart, and liver tissues and the kidney and spleen on the last day (a day before death) (8).
A study by Derouin and Garin on the RH strain infection reported the highest parasite burden in the lungs and blood (22). In addition, Jauregui et al. diagnosed toxoplasmosis in the infected tissues of pigs and mice, using qPCR; however, no reliable results were reported in larger animals due to an error at the time of sampling (23). Also, Zenner et al. reported the highest level of parasites in the lungs, liver, brain, and blood (24). In the present study on various tissues of infected mice, the maximum parasite count was detected in both groups at different intervals in the blood and heart, respectively. Twenty-four hours after intraperitoneal tachyzoite inoculation, the highest parasite burden was observed in the liver and kidney tissues of both groups. The increased parasite burden in these tissues is most likely due to intraperitoneal inoculation with tachyzoite and primary adjacency of these tissues, which was similar in both groups.
Based on the present results, the highest level of parasites was observed in the blood, heart, and lungs on the final day (death time). The observed increase of parasite count in the blood was probably due to the adjacency of tissues and contribution of blood flow to parasite transmission to other tissues. The kinetics and parasite burden showed similar distribution patterns in the groups at the time of death; a difference was only observed between the groups during movement to reach the peak of parasite load.
Overall, the results suggest that T. gondii can be transmitted within the first 24 hours of infection or even earlier, as it quickly activates in the body. In the present study, as well as similar research, tachyzoites were detected within 1 day after infection in the brain and eyes. It is remarkable that protozoa can pass through the blood-brain barrier in a very limited amount of time after infecting the mice.
Considering the substantial parasite load in the genitals and presence of parasites in the body, the possibility of sexual transmission is also suggested, and further research is necessary in this area. The qPCR assay is recommended for the accurate and sensitive diagnosis of infection in high-risk individuals, such as pregnant women and people with immunodeficiency; also, it can be applied in research activities to determine the parasite load. In addition, knowledge about the distribution of T. gondii is of utmost importance, as the majority of pharmaceutical studies have been performed on mouse in vivo models. The present study provides significant information on targeting the most suitable tissues for evaluating the effectiveness of new vaccines and drugs against T. gondii.