1. Background
In December 2019, coronavirus disease 2019 (COVID-19) appeared in Wuhan, China, rapidly disseminated worldwide and became a pandemic (1). Since then, over 759 million confirmed cases of COVID-19 and more than 6.8 million deaths have been reported (2). The most common signs and symptoms of COVID-19 are related to the respiratory system, including cough, coryza, sore throat, dyspnea, and hypoxemia, as well as systemic inflammatory response, including fever and chills, myalgia, and fatigue (3).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has neurotropic properties and can cause neurological diseases such as epilepsy, disturbed consciousness, and viral encephalitis (4). One of the neurologic involvement aspects of SARS-CoV-2 is the sudden loss of smell and taste, called anosmia and dysgeusia (5). According to a study by Spinato et al. performed on 347 patients with COVID-19, 64.4% of all patients reported an altered sense of smell. The olfactory disturbance was more frequent in women (72.4%) than in men (55.7%). The onset of olfactory symptoms could be before, simultaneously, or after the beginning of other disease symptoms (6).
According to the systematic review performed by Mutiawati et al., the global prevalence of anosmia in patients with COVID-19 was 38.2%, being 10.2 folds higher in these patients than in other COVID-19-like illnesses (7). According to the study of Algahtani et al., the most prevalent symptoms in a post-acute syndrome of COVID-19 were anosmia (33.8%) and ageusia (26.4%) (8). Anosmia was more prevalent in women and youth (5, 8).
It has been shown that SARS-CoV-2 uses ACE2 as a receptor to enter and infect the cells in the respiratory tract (9). On the other hand, human olfactory mucosa expresses ACE2. As known, ACE2 is expressed in support cells, stem cells, and perivascular cells. Infecting cells by SARS-CoV-2 and subsequent inflammation may account for olfactory disturbance in patients with COVID-19 (10, 11).
2. Objectives
There is little information about prognostic value in patients with COVID-19. Prior studies on the correlation between the severity of olfactory involvement and the severity of COVID-19 were inconclusive, while the presence of olfactory involvement is associated with less severe COVID-19 (12-14). Therefore, we designed this study with a larger sample size than prior studies to assess the association between the severity of the olfactory disturbance and the COVID-19 severity.
3. Methods
In this prospective cohort study, we evaluated 390 patients with COVID-19 referred to Taleghani Hospital, Tehran, Iran, between March 2021 and March 2022. Inclusion criteria were a definitive diagnosis of COVID-19 by a positive PCR test for COVID-19 or a positive chest CT scan indicating typical pulmonary involvement of COVID-19 without a history of previous olfactory disorders. Patients with at least one positive test mentioned before entered the study. Exclusion criteria were being unwilling to keep participating, incomplete clinical information of the disease and olfactory disturbance, and inability to assess the olfactory function.
Data on demographic information and past medical history, including diabetes and hypertension, were gathered. Data on symptoms and severity of COVID-19, according to clinical factors of disease at the time of hospitalization, were evaluated by the physician in charge of the patient and recorded as an ordinal variable (1: Mild, 2: Moderate, 3: Severe and 4: Critical). The course of the disease, such as recovery from disease and discharge, ICU admission without intubation, intubation, and death, was assessed and recorded. Patients who died from the disease were excluded from other categories based on their intubation or ICU admission status. The presence of olfactory disturbance and its severity when visiting the hospital was asked from patients and recorded as an ordinal variable (1: Anosmia, 2: Moderate hyposmia, or 3: Normosmia).
Patients were categorized according to their age (young age group: < 25 years old, middle age group: > 25 and < 65 years old, and old age group: > 65 years old). Also, patients were categorized according to their BMI (underweight: BMI < 19.9, normal weight: 20 ≤ BMI ≤ 24.9, overweight: 25 ≤ BMI, obese: 30 ≤ BMI ≤ 34.9, moderately obese: 35 ≤ BMI).
The study obtained approval from the ethics committee under the reference number IR.SBMU.MSP.REC.1400.776, and all participants provided informed consent before enrolling in the study.
Data were analyzed using Stata version 14 software. Numeric variables are presented as mean (SD), and categorical variables as percentages. The Spearman correlation test was used to assess the association between COVID-19 severity and olfactory disturbance severity. Also, the association between COVID-19 severity and other numeric or ordinal variables was evaluated using the Spearman correlation method. Ordinal logistic regression was used to investigate the association between COVID-19 severity and other variables. After performing univariate logistic regression, variables were entered into multiple logistic regression to evaluate the predicting factors of olfactory disturbance severity in patients with COVID-19.
4. Results
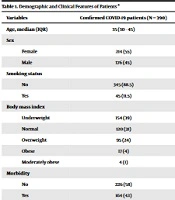
A total of 390 patients entered the study, and their data were analyzed. The median age was 36 years, with an interquartile range (IQR) of 30 - 45. Fifty-five percent (n = 214) of the patients were female. Smokers consisted of 11.5% (n = 45) of the patients. Forty-two percent (n = 164) had a comorbidity. Among them, diabetes mellitus and hypertension were present in 3.6% (n = 14) and 14.4% (n = 65) of the patients, respectively. In terms of the body mass index, 5.4% (n = 21) were obese (including all categories of obesity), 24.4% (n = 95) were overweight, and the remaining had a normal weight or were underweight. The complete demographic information is demonstrated in Table 1.
| Variables | Confirmed COVID-19 Patients (N = 390) |
|---|---|
| Age, median (IQR) | 35 (30 - 45) |
| Sex | |
| Female | 214 (55) |
| Male | 176 (45) |
| Smoking status | |
| No | 345 (88.5) |
| Yes | 45 (11.5) |
| Body mass index | |
| Underweight | 154 (39) |
| Normal | 120 (31) |
| Overweight | 95 (24) |
| Obese | 17 (4) |
| Moderately obese | 4 (1) |
| Morbidity | |
| No | 226 (58) |
| Yes | 164 (42) |
| Diabetes mellitus | |
| No | 376 (96) |
| Yes | 14 (4) |
| Hypertension | |
| No | 334 (86) |
| Yes | 56 (14) |
Demographic and Clinical Features of Patients a
The severity of COVID-19 at admission and its progression is shown in Table 2. Fifteen percent (n = 58) of the patients had mild COVID-19. Seventy-four (18.5%) patients had severe and critical forms of COVID-19. The others had moderate COVID-19 (66.2%, n = 258). Most (88%) of the patients recovered from COVID-19 (n = 345). Thirty-three patients (8.5%) were admitted to the ICU without intubation. One percent of the patients needed intubation (n = 3). Finally, 2.3% of the patients died due to complications of COVID-19 (n = 9). The severity of olfactory disturbance is also shown in Table 2. Forty-four percent of the patients (n = 171) had complete anosmia at admission. Thirty-two percent (n = 126) did not report any olfactory disturbance to the hospital at admission. All other patients (24%, n = 93) experienced some degree of olfactory disturbance.
| Variables | Frequency (%) |
|---|---|
| Severity of COVID-19 at admission | |
| Mild | 58 (15) |
| Moderate | 258 (66) |
| Severe | 68 (17) |
| Critical | 6 (1.5) |
| Progression of the disease | |
| Recovery | 345 (88) |
| ICU admission | 33 (9) |
| Intubation | 3 (1) |
| Death | 9 (2) |
| Severity of olfactory disturbance at admission | |
| Anosmia | 171 (44) |
| Moderate hyposmia | 93 (24) |
| Normal | 126 (32) |
Severity and Prognosis of COVID-19 and Olfactory Disturbance Among Patients
Table 3 shows the Spearman correlation coefficients and the corresponding P-values. Among all variables tested, the severity of COVID-19 had a weak but statistically significant negative association with the severity of olfactory disturbance (r = -0.132, P-value = 0.009). Smoking and diabetes mellitus also had a weak but statistically significant association with the severity of olfactory disturbance (r = 0.101, P-value = 0.046 and r = 0.115, P-value = 0.024, respectively).
| Anosmia, No. (%) | Moderate Anosmia, No. (%) | Normal, No. (%) | Spearman's Correlation Coefficient | P-Value | |
|---|---|---|---|---|---|
| Age group | - 0.008 | 0.872 | |||
| Young age | 10 (5) | 3 (3) | 6 (5) | ||
| Middle age | 156 (92) | 88 (97) | 117 (93) | ||
| Old age | 5 (3) | 0 (0) | 3 (2) | ||
| Sex | 0.033 | 0.514 | |||
| Male | 73 (43) | 45 (48) | 58 (46) | ||
| Female | 98 (57) | 48 (52) | 68 (54) | ||
| Smoking status | 0.101 | 0.046 | |||
| No | 145 (85) | 84 (90) | 116 (92) | ||
| Yes | 26 (15) | 9 (10) | 10 (8) | ||
| Body mass index | - 0.080 | 0.113 | |||
| Underweight | 78 (46) | 27 (29) | 49 (39) | ||
| Normal | 49 (29) | 32 (34) | 39 (31) | ||
| Overweight | 40 (23) | 25 (27) | 30 (24) | ||
| Obese | 2 (1) | 9 (10) | 6 (5) | ||
| Moderately obese | 2 (1) | 0 (0) | 2 (2) | ||
| Hypertension | 0.020 | 0.693 | |||
| No | 146 (85) | 78 (84) | 110 (87) | ||
| Yes | 25 (15) | 15 (16) | 16 (13) | ||
| Diabetes | 0.115 | 0.024 | |||
| No | 162 (95) | 88 (95) | 126 (100) | ||
| Yes | 9 (5) | 5 (5) | 0 (0) | ||
| Progression | 0.171 | 0.001 | |||
| Recovery | 157 (92) | 89 (96) | 99 (79) | ||
| ICU admission | 14 (8) | 4 (4) | 15 (12) | ||
| Intubation | 0 (0) | 0 (0) | 3 (2) | ||
| Death | 0 (0) | 0 (0) | 9 (7) | ||
| COVID-19 severity | - 0.132 | 0.009 | |||
| Mild | 27 (16) | 20 (21) | 11 (9) | ||
| Moderate | 117 (68) | 61 (66) | 80 (63) | ||
| Severe | 27 (16) | 12 (13) | 29 (23) | ||
| Critical | 0 (0) | 0 (0) | 6 (5) |
Association of Olfactory Disturbance Severity with Demographic Characteristics, Medical Comorbidities, and COVID-19 Severity
In univariate ordered logistic regression, smoking had a statistically significant predictive value for olfactory disturbance severity (OR = 1.9, P-value = 0.046, 95%CI = 1.0 - 3.4). In this model, patients with diabetes mellitus were more likely to develop olfactory disturbance than patients without diabetes mellitus (OR = 3.0, P-value = 0.039, 95%CI = 1.1 – 8.7). Furthermore, obesity and severe COVID-19 had borderline significance in predicting the severity of olfactory disturbance (OR = 0.4, P-value = 0.056, 95%CI = 0.2 – 1.0, and OR = 0.6, P-value = 0.063, 95%CI = 0.3 – 1.0, respectively). In multiple logistic regression, obesity was a statistically significant negative predictor of developing olfactory disturbance in patients with COVID-19 (OR = 0.4, P-value = 0.043, 95%CI = 0.1 – 0.9). In this model, diabetes mellitus was a statistically significant positive predictor of developing olfactory disturbance (OR = 3.3, P-value = 0.029, 95%CI = 1.1 – 9.9). Patients with severe and critical COVID-19 were less likely to develop olfactory disturbance (OR = 0.5, P-value = 0.035, 95%CI = 0.2 – 0.9). In the latter model, smoking had no significant predictive value (OR = 1.7, P-value = 0.081, 95%CI = 0.9 – 3.3). Table 4 shows the results of univariate and multivariate ordered logistic regression models to determine the predictors of olfactory disturbance severity.
| Univariate Logistic Regression | Multivariate Logistic Regression | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | P-Value | 95% CI | Odds Ratio | P-Value | 95% CI | |
| Age group | ||||||
| Young age | Reference | Reference | ||||
| Middle age | 0.8 | 0.581 | 0.3 – 1.9 | 0.8 | 0.816 | 0.3 – 2.3 |
| Old age | 1.2 | 0.833 | 0.2 – 6.6 | 1.7 | 0.539 | 0.3 – 10.2 |
| Gender | ||||||
| Male | Reference | Reference | ||||
| Female | 1.1 | 0.513 | 0.8 - 1.6 | 1.1 | 0.723 | 0.7 – 1.6 |
| BMI | ||||||
| Underweight | Reference | Reference | ||||
| Normal | 0.8 | 0.271 | 0.5 – 1.2 | 0.8 | 0.470 | 0.5 – 1.3 |
| Over weight | 0.8 | 0.401 | 0.5 – 1.3 | 0.8 | 0.410 | 0.5 – 1.3 |
| Obese | 0.4 | 0.056 | 0.2 – 1.0 | 0.4 | 0.043 | 0.1 – 0.9 |
| Moderately obese | 0.7 | 0.683 | 0.1 – 5.0 | 0.7 | 0.779 | 0.1 – 5.6 |
| Smoking | ||||||
| No | Reference | Reference | ||||
| Yes | 1.9 | 0.046 | 1.0 – 3.4 | 1.7 | 0.081 | 0.9 – 3.3 |
| Hypertension | ||||||
| No | Reference | Reference | ||||
| Yes | 1.1 | 0.695 | 0.7 – 1.9 | 1 | 0.943 | 0.6 – 1.8 |
| Diabetes mellitus | ||||||
| No | Reference | Reference | ||||
| Yes | 3 | 0.039 | 1.1 – 8.7 | 3.3 | 0.029 | 1.1 – 9.9 |
| COVID-19 severity | ||||||
| Mild | Reference | Reference | ||||
| Moderate | 0.78 | 0.366 | 0.46 – 1.32 | 0.7 | 0.205 | 0.4 – 1.2 |
| Severe and critical | 0.45 | 0.015 | 0.23 – 0.85 | 0.5 | 0.035 | 0.2 – 0.9 |
Univariate and Multivariate Ordered Logistic Regression to Determine the Predictors of Olfactory Disturbance Severity
5. Discussion
Olfactory disturbance, including anosmia and hyposmia, is one of the most frequent signs or symptoms of COVID-19. Nasal neuroepithelial cells express high levels of essential receptors, i.e., ACE2, for SARS-CoV-2 entry (15, 16). After the entry of the virus into nasal neuroepithelial cells, an inflammatory response could lead to improper functioning of these neurons and cause olfactory disturbance (17).
This study found that patients with severe COVID-19 are slightly less likely to develop olfactory disturbance. In line with our findings, the study by Yan et al. revealed that admission due to COVID-19 was strongly correlated with an intact sense of smell and taste. Additionally, patients who experienced complete anosmia had significantly lower admission rates and, in most cases, were managed in outpatient settings (18). According to the study by Talavera et al., patients with anosmia and severe olfactory disturbance had lower ICU admission and mortality (19).
Ultimately, the meta-analysis study by Purja et al. demonstrated that COVID-19 patients who manifest anosmia have better outcomes and milder COVID-19 severity than others (20). However, they did not further assess the association of the extent of olfactory disturbance with COVID-19 severity.
Evidence suggests that the serum IL-6 is significantly lower in COVID-19 patients with anosmia than those without anosmia. It has been hypothesized that patients who generate a well-established antiviral response on the olfactory epithelium, which is the first line of defense against virus attack, experience a milder disease. However, because of the olfactory epithelium involvement, olfactory disturbance and loss of smell will happen (21-23).
In this study, we did not find any significant association between gender and the severity of olfactory disturbance. On the other hand, the study by Lee et al. found that either anosmia or ageusia was significantly more prevalent in females than males (24). Furthermore, in the study by Najafloo et al., females were more likely to experience severe forms of olfactory disturbance; meanwhile, they were less likely to have severe COVID-19 than males. They proposed that lower ACE2 expression in the nasal epithelium and a stronger female immune system than that of male patients could partly explain these findings (25).
We found that obesity is an independent predictor of less severe types of olfactory disturbance. However, we did not find any significant association between the normal or underweight status of COVID-19 patients and the severity of olfactory disturbance. Obesity exacerbates the SARS-CoV-2 infection. Adipose tissue is an important source of proinflammatory cytokines, and obese people experience a chronic and low-grade inflammatory state. Consequently, it enhances the characteristic COVID-19 cytokine storm when the virus attacks (26). The cytokine storm is responsible for the severe forms of COVID-19. Therefore, it is predictable that obese patients experience less severe olfactory disturbance. According to the study by Khan et al., obesity was associated with lower oro-naso-sensory perception, and obese patients with COVID-19 were less likely to experience olfactory disturbance (27).
We also found that patients with COVID-19 who had diabetes mellitus were 3.3 times more likely to have severe olfactory disturbance and anosmia. In agreement with our findings, the study by Zhao et al. demonstrated that type two diabetes mellitus was associated with an increased risk of olfactory disturbance. They used an animal (mice) model to demonstrate underlying pathology. In type two diabetes mellitus mice, there was an upregulated expression of ACE2 in the nasal mucosa (28). Also, mice with type two diabetes mellitus had altered lymphocyte components in the nasal-associated lymphoid tissue (NALT), which weakened the first line of defense in the nasal mucosa. According to a one previous study, patients with diabetes mellitus and COVID-19 are more likely to demonstrate severe types of olfactory disturbance, probably because of underlying neurologic damage due to diabetes mellitus (29).
We designed a prospective cohort study with a relatively large sample size and evaluated the olfactory disturbance during the disease, hence eliminating recall bias. The subjective report of olfactory disturbance was the main limitation of this study.
In conclusion, we evaluated the olfactory disturbance in COVID-19 and its associated factors. We found that patients with severe COVID-19 demonstrated milder forms of olfactory disturbance. Therefore, the severity of olfactory disturbance can be used as a prognostic factor in COVID-19. It is important to closely monitor patients who exhibit mild olfactory disturbance because they are at risk of a more severe form of COVID-19.