1. Background
The skin is commonly affected by metabolic, vascular, neurological, neoplastic, nutritional disorders, and, above all, adverse drug reactions and in various infectious and contagious diseases, including viral ones (1, 2). Several cutaneous manifestations of COVID-19 have been described since its inception. There are several skin changes reported to be associated with COVID-19, and to describe them in a more didactic way; it is important to propose a classification based on their morphological and evolutionary characteristics, which have already been discussed in the medical literature (3-5).
Dermatological manifestations linked to changes in behavior and habits during the pandemic have been observed, which, in different ways, can facilitate risk exposures for skin lesions and, consequently, the emergence of skin diseases. Contact dermatitis triggered by personal protective equipment as well as hygiene and antisepsis products widely used during the pandemic are very illustrative examples (6-11).
Other effects of the pandemic that may have affected people's health have been social distancing measures, lockdowns, and interruption of public services in outpatient clinics and medical offices; these have caused delays in the diagnosis and treatment of various diseases (12), such as delays in the detection of melanomas (13, 14).
The direct relationship between SARS-CoV-2 and cutaneous manifestations in patients with COVID-19 can be evidenced by biopsies of skin lesions (15-17); In the analysis by immunohistochemistry, and also in the deposit of complement, glycoproteins, or genomic material of the virus in the examined tissue (18, 19) as well as by electronic microscopy (20), several cutaneous manifestations have been histopathologically described as a consequence of an immunoreactive response to COVID-19 (21).
In the scenario of COVID-19, pharmacodermias became common with the use of drugs, with or without proven efficacy. In the presence of COVID-19, the occurrence of drug hypersensitivity was 11.4% of patients (22), and > 20% among Italian patients (23). With the increase in self-medication during the COVID-19 pandemic, it was expected that many adverse reactions would occur (24-26). In addition, drug-triggered eruptions can be, in most cases, indistinguishable from the skin rash caused by COVID-19 (27). Adverse events of vaccines against SARS-CoV-2 occur mainly due to the activation of the immune system, not necessarily being caused by allergic reactions (28). The vaccine reaction most described in the literature was the reaction at the injection site (29). Most of the published works on adverse events of vaccines against COVID-19 refer to the immunizing mRNA, probably because this vaccine format has been applied mainly to populations from countries with a large volume of scientific publications in the medical field.
2. Methods
We aimed to propose a classification of dermatological manifestations related to either COVID-19 or vaccination against SARS-CoV-2. This was a descriptive observational study of dermatological clinical characteristics of patients with cutaneous manifestations related to the COVID-19 pandemic, with cutaneous lesions arising during or after the manifestation of COVID-19 or post-vaccination against SARS-CoV-2.
The study was performed at two reference services of dermatology, one public and one private, both located in the Central Brazil Region. All included patients were assisted in outpatient offices and/or by telemedicine care from July 2020 to July 2022. All patients seeking the two reference services with any cutaneous manifestation and reporting a recent diagnosis of COVID-19 or recent vaccination against SARS-CoV2 or use of protective measures against the new coronavirus were included in the study. The studied sample was conveniently selected among sequential patients seen during the evaluation period and who agreed to participate in the study.
Confirmation of COVID-19 was made by a positive result in RT-PCR or rapid antigen tests or in antibody tests against SARS-CoV-2. Vaccination certificates were requested to prove previous vaccination against SARS-CoV-2. Photos of the skin lesions were taken using smartphone digital cameras, mainly for patients seen via teleconsultation, for verification and characterization of the lesions. Dermatological lesions that appeared within the period of symptoms of COVID-19, in its convalescence, or after specific vaccination were considered incident cutaneous manifestations among the study patients. When necessary, skin biopsies were obtained for diagnostic confirmation and treatment of patients.
After the clinical and laboratory characterization, the cutaneous manifestations were classified as incident or acute dermatological lesions in patients, either resulting from COVID-19 or after vaccination against SARS-CoV-2. Lesions were classified into groups of hypotheses etiological, based on the analysis of information from anamnesis, physical examination, and results of laboratory tests.
Microsoft Excel® software was used for data tabulation. Absolute and relative frequency distribution tables were created to summarize the main study variables. This study was approved by the Research Ethics Committee of the Hospital Universitário Júlio Müller (CEP/HUJM) by document number 4,733,645 of May 25, 2021. The informed consent form was signed by all included patients.
3. Results
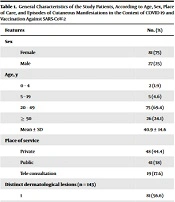
From July 2020 to July 2022, 114 patients who had dermatological manifestations temporally related to COVID-19 and/or incidents after receiving a vaccine against SARS-CoV-2 were eligible for this study. Of these, six patients were not included in the analysis because four of them were unable to maintain regular contact with the study team, one other because the diagnosis of scabies was confirmed as the cause of the cutaneous manifestation, and the other because of refusal to participate in the study. Thus, the results of 108 patients were analyzed, of which 48 (44.4%) were treated in the private service, 41 (38%) in the public service, and 19 (17.6%) by telemedicine care. The sample consisted of 81 (75%) female patients and 27 (25%) male patients, with a mean age (SD) of 40.9 (14.6) years, the youngest being 8 months and the oldest 82 years at the time of consultation. In these 108 patients, 143 different dermatological manifestations were observed, being identified as a single dermatological diagnosis in 81 patients and as two different dermatological diagnoses in 20 patients; six patients had three different dermatological diagnoses, and one patient had four different dermatological diagnoses (Table 1).
| Features | No. (%) |
|---|---|
| Sex | |
| Female | 81 (75) |
| Male | 27 (25) |
| Age, y | |
| 0 – 4 | 2 (1.9) |
| 5 – 19 | 5 (4.6) |
| 20 – 49 | 75 (69.4) |
| ≥ 50 | 26 (24.1) |
| Mean ± SD | 40.9 ± 14.6 |
| Place of service | |
| Private | 48 (44.4) |
| Public | 41 (38) |
| Tele consultation | 19 (17.6) |
| Distinct dermatological lesions (n = 143) | |
| 1 | 81 (56.6) |
| 2 | 20 (28) |
| 3 | 6 (12.6) |
| 4 | 1 (2.8) |
| COVID-19 episodes per patient (n = 108) a | |
| 0 | 12 (11.1) |
| 1 | 89 (82.4) |
| 2 | 6 (5.6) |
| 3 | 1 (0.9) |
| Event causing skin manifestation | |
| COVID-19 | 83 (76.8) |
| Vaccine | 15 (13.9) |
| COVID-19 and vaccine | 7 (6.5) |
| Socio-sanitary context of the pandemic | 3 (2.8) |
a 12 patients presented vaccine reaction, with no history of COVID-19.
Eighty-three patients (76.8%) had a reaction temporally linked to the COVID-19 condition. In four of them, there was a recurrence of COVID-19, and in two of these cases, the patients had the same dermatological condition in both infections. In one patient, the dermatological manifestation of the first episode of COVID-19 was different from that presented in the second infection. In the other patient, the cutaneous manifestation only occurred during the second episode of COVID-19. One patient had recurrent and confirmed COVID-19 in three episodes but only had a dermatological reaction in one of them. Three patients (2.8%) had skin conditions related to the pandemic, with their dermatological manifestations being an indirect consequence of COVID-19 (Table 1).
Fifteen (13.9%) patients had only a post-vaccine reaction, one of which had the same dermatological manifestation after two doses of the vaccine, and another three had already presented COVID-19 without any dermatological manifestation in the course of the disease but presented a dermatological condition only after receiving the vaccine against SARS-CoV-2.
Four patients (3.7%) had dermatological manifestations during COVID-19 and also after the vaccination against SARS-CoV-2 at different times. Another three patients (2.8%) had a skin reaction after vaccination, but they were in their first days of symptoms of COVID-19 at the same time as vaccination. For these cases, it was unclear whether the dermatological reactions were triggered by COVID-19, by the vaccine, or by both. One of them was classified as a peri-infectious immune-inflammatory reaction by COVID-19, as the monomorphic rash reaction observed in this patient was similar to the other patterns seen among the other patients within this classification. The other two were classified as a post-vaccination skin reaction, and in one of them, both COVID-19 and vaccination against SARS-CoV-2 may have caused the skin lesions of pharmacodermia; in the other case, the skin condition of lichen plan had already been described as a possible post-vaccination reaction (Table 1).
Five groups of probable etiologies for the dermatological lesions identified in this study were established according to the following criteria:
(I) Lesions that presented morphological, semiotic, clinical characteristics or even with references available in the medical literature of their immune or autoimmune behavior during infections or possibly associated with viral infectious action were classified as peri-infectious and post-infectious immune-inflammatory reactions related to COVID-19.
(II) Dermatological lesions prior to COVID-19, whose symptomatology or intensity was affected by the disease, were classified as an exacerbation, by COVID-19, of pre-existing dermatological diseases in the patient.
(III) Skin lesions related to any of the preventive measures imposed to reduce the transmission of the coronavirus and which, after clinical evaluation, could not be explained by an immunoinflammatory reaction from infections, pharmacodermia, or vaccine reaction, were classified as cutaneous manifestations motivated by the socio-sanitary context of the pandemic.
(IV) Incident skin lesions after using drugs known to be associated with the presented skin reaction, excluding other possible causes that could justify the lesion, were classified as adverse drug reactions to the treatment of COVID-19.
(V) Incident skin lesions within a period of 30 days after vaccination against the SARS-CoV-229 virus, whereas another etiology was excluded, were classified as adverse skin reactions to SARS-CoV-2 vaccines.
Table 2 presents the etiological diagnoses made for the 143 dermatological lesions presented by the patients in the study, grouped according to the criteria for the formulation of etiological hypotheses for skin manifestations in these patients.
| Classification and Diagnosis | No. |
|---|---|
| 1. Peri-infectious and post-infectious immune-inflammatory reactions related to COVID-19 | |
| Telogen effluvium | 47 |
| Urticaria | 13 |
| Monomorphic eruption | 4 |
| Localized skin rash | 4 |
| PIDS | 2 |
| Acne eruption (without corticosteroid) | 2 |
| Lip herpes | 2 |
| Alopecia areata | 1 |
| Dermographism | 1 |
| Early ecchymosis | 1 |
| Erythema multiforme | 1 |
| Vesicular eruption | 1 |
| Vesicular and pustular eruption | 1 |
| Furunculosis | 1 |
| Annular granuloma | 1 |
| Livedo reticularis | 1 |
| Perniosis (COVID toes) | 1 |
| Pityriasis rosea | 1 |
| Dysautonomic syndrome (hyperhidrosis) | 1 |
| Sweet's Syndrome | 1 |
| Nail fragility syndrome | 1 |
| Xeroderma | 1 |
| 2. Exacerbation, by COVID-19, of pre-existing dermatological diseases in the patient | |
| Vitiligo reactivation | 3 |
| Secondary exacerbation of syphilis | 1 |
| Skin desquamation | 1 |
| Raynauld's phenomenon | 1 |
| 3. Adverse drug reactions from the treatment of COVID-19 | |
| Acne eruption | 12 |
| Urticaria | 2 |
| DRESS | 2 |
| Pharmacodermia (others) | 2 |
| Red half-moon nail sign | 2 |
| 4. Skin manifestations motivated by the socio-sanitary context of the pandemic | |
| Mask contact dermatitis | 1 |
| Pressure alopecia | 1 |
| Matting of hair | 1 |
| 5. Adverse skin reactions from SARS-CoV-2 vaccines | |
| Urticaria | 5 |
| Injection local reaction | 3 |
| Lichen planus/lichenoid eruption | 3 |
| Exacerbation of venous ulcers | 3 |
| SDRIFE | 2 |
| Pharmacodermia (others) | 2 |
| Vitiligo | 1 |
| PIDS | 1 |
| Hailey-Hailey exacerbation | 1 |
| Acute systemic lupus erythematosus | 1 |
| Lichenoid pityriasis | 1 |
| Psoriasis | 1 |
| Exacerbation of leprosy reaction | 1 |
Abbreviations: PIDS, persistent, intermittent delayed swelling, late adverse reaction to hyaluronic acid fillers; DRESS, Drug rash with eosinophilia and systemic symptoms; SDRIFE, symmetrical drug-related intertriginous and flexural exanthema.
4. Discussion
The peri-infectious and post-infectious immune-inflammatory reactions related to COVID-19 were the most observed cutaneous manifestations in our study, with 89 distinct dermatological manifestations, the most common being peri/post-infectious telogen effluvium, followed by urticarial reactions and urticaria. Other manifestations included monomorphic eruptions, skin rash predominantly on the face and neck but also occurring on the limbs and flanks, late intermittent and persistent edema due to filler (PIDS), acne eruptions without the use of corticosteroids, labial herpes, alopecia areata, erythema multiforme, granuloma annulare, dysautonomic syndrome with generalized hyperhidrosis, pityriasis rosea, Sweet's syndrome, early ecchymosis, vesicular eruption, a vesicular and pustular eruption, dermographism, livedo reticularis, xeroderma, perniosis, weak nail syndrome, and furunculosis.
In some patients, pre-existing dermatologic conditions were exacerbated, most likely due to the inflammatory and/or immune response triggered by COVID-19. Cases of reactivation of vitiligo, pruridermia, and skin spots with exacerbation of secondary syphilis and worsening of symptoms of a patient with systemic scleroderma were observed, classified as exacerbation by COVID-19 of pre-existing dermatological diseases.
Adverse drug reactions to the treatment of COVID-19 were expected events, especially due to the large number of self-medications consumed by the general population during the pandemic, often encouraged by the media and social networks (24-26, 30, 31). However, among the cases analyzed here, most pharmacodermal reactions were caused by medications usually used to treat the inflammatory response to COVID-19.
Not all patients who required dermatological evaluation in this case series had dermatologic manifestations directly resulting from COVID-19. Some of the observed skin lesions arose as a result of the socio-sanitary context of the pandemic, such as hygiene habits, attitudes, and social behaviors; these were classified as cutaneous manifestations motivated by the socio-sanitary context of the pandemic.
Adverse skin reactions to vaccines against SARS-CoV-2 were observed. Some patients presented more than one dermatological manifestation, and others presented both a cutaneous reaction due to the disease and to the vaccine. Such reactions were commonly type I or IV allergic reactions, which can be triggered by the immunizing antigen, agents used as adjuvants, preservatives, stabilizers, or even residues of antimicrobial agents or of the culture medium used for vaccine development (32). In addition, localized hypersensitivity reactions may possibly occur due to polyethylene glycol, present as a vaccine vehicle. In vaccine studies against SARS-CoV-2, in addition, to type I and IV hypersensitivity, some patients also had a type III immune complex reaction, known as the Arthus phenomenon (21).
Some limitations of this study should be highlighted: the small sample size, the entire assessment performed by a single specialist, and the lack of immunohistochemical and metabolic tests to verify the etiological mechanism of the found cutaneous lesions. For this reason, further studies performed by other skin disease experts are necessary to validate the proposed classification.
4.1. Conclusions
Cutaneous manifestations related to COVID-19 or to vaccines against SARS-CoV-2 can be classified into (I) peri-infectious and post-infectious immune-inflammatory reactions related to COVID-19; (II) exacerbation, by COVID-19, of pre-existing skin diseases in the patient; (III) cutaneous manifestations resulting from the socio-sanitary context of the pandemic; (IV) adverse drug reactions from the treatment of COVID-19; (V) adverse skin reactions from SARS-CoV-2 vaccines. Although further studies with appropriate methodology are needed to validate the classification proposed here, it is expected that this classification will serve as an aid to medical professionals for more assertive decision-making in situations where the patient presents with cutaneous manifestations associated with the COVID-19 or after active immunization against SARS-CoV-2.