1. Background
Opioid addiction is a significant health and social issue of the current century. It not only causes severe physical and psychological harm to individuals but also leads to social problems such as divorce, delinquency, prostitution, and unemployment, imposing substantial economic burdens. Approximately 90% of opioid combination users also suffer from concomitant psychological disorders, most commonly major depression, alcoholism, antisocial personality disorder, and anxiety (1).
In late December 2019, a new strain of coronavirus was identified, leading to the COVID-19 pandemic, which has affected all aspects of people’s lives (2-4). Individuals with substance use disorders appear to be more vulnerable to the serious consequences of the pandemic (5). Addiction is particularly significant in this context for two reasons: First, the living conditions of addicted individuals (e.g., poor hygiene) increase their risk of infection, and second, their heightened vulnerability to contracting the disease. Recently, however, a misunderstanding has spread through social media and communities, falsely claiming a protective effect of opioid combination consumption against COVID-19, thereby encouraging addiction (6).
In contrast, multiple studies have identified opioid combination consumption as a risk factor for viral infections, especially pulmonary infections. This is attributed to various predisposing factors, such as cardiopulmonary disease, mucosal dysfunction, weakened immunity, adverse health behaviors, limited access to healthcare services, poor living conditions, and other disabilities, all of which increase the risk of COVID-19 among addicts (7). Additionally, individuals with pre-existing respiratory and cardiac conditions are considered high-risk populations for COVID-19 and acute respiratory complications, leading to higher COVID-19-related mortality rates in these groups (8, 9). In fact, due to pre-existing cardiac and respiratory issues, individuals with addictions are more susceptible to contracting and succumbing to COVID-19.
Moreover, those who use opioid combinations to self-treat conditions like COVID-19 are at risk of fatal drug overdoses, which can cause respiratory failure, hypoxemia, and subsequent cardiopulmonary and neurological complications, exacerbating the outcomes of COVID-19 (10).
Mahdavi and Aliramezany refuted the hypothesis that addiction could reduce the risk of contracting COVID-19 (6). Another review in 2021 identified four reasons for increased mortality in addicts with COVID-19: (1) Elevated interferon production, (2) increased risk of pulmonary edema, (3) heightened clot formation rates, and (4) overexpression of angiotensin-converting enzyme 2 (ACE2) (11). However, to the best of our knowledge, studies on this topic remain limited.
2. Objectives
Therefore, the objective of this study was to identify the prognostic factor of in-hospital mortality in confirmed COVID-19 patients with opioid combination consumption in southern Iran.
3. Methods
This retrospective cross-sectional study, conducted between September 2020 and March 2021, included all adult patients [> 14 years old (12)] hospitalized with a confirmed diagnosis of COVID-19 at Shiraz Shahid Faghihi Hospital, affiliated with Shiraz University of Medical Sciences. This hospital is one of the primary centers for managing hospitalized COVID-19 patients. Patients were classified as opioid substance users based on their reported history and records in their medical files. Patients with incomplete or missing medical records were excluded from the study.
Data were extracted from the patients' medical files and recorded in a structured data collection form comprising three main sections: (1) Demographic characteristics (e.g., age, gender, comorbidities, smoking history), (2) clinical and paraclinical findings, and (3) patient outcomes.
Data were entered into SPSS version 16.0 for Windows and subsequently analyzed. The Independent Sample t-test or Mann-Whitney test was used for comparing continuous variables between two groups. Chi-square and Fisher’s exact tests were applied for categorical proportions. For univariate logistic regression analysis, each variable was entered individually. Variables with a P < 0.2 in the univariate analysis were included in the multivariate logistic regression analysis, using the Forward Stepwise method to identify predictive factors for in-hospital mortality. Odds ratios (OR) were calculated and reported.
Results were presented as mean ± standard deviation (SD) for continuous variables and summarized as numbers (percentages) for categorical variables. A two-sided P-value of less than 0.05 and a 95% confidence interval (CI) were considered statistically significant.
4. Results
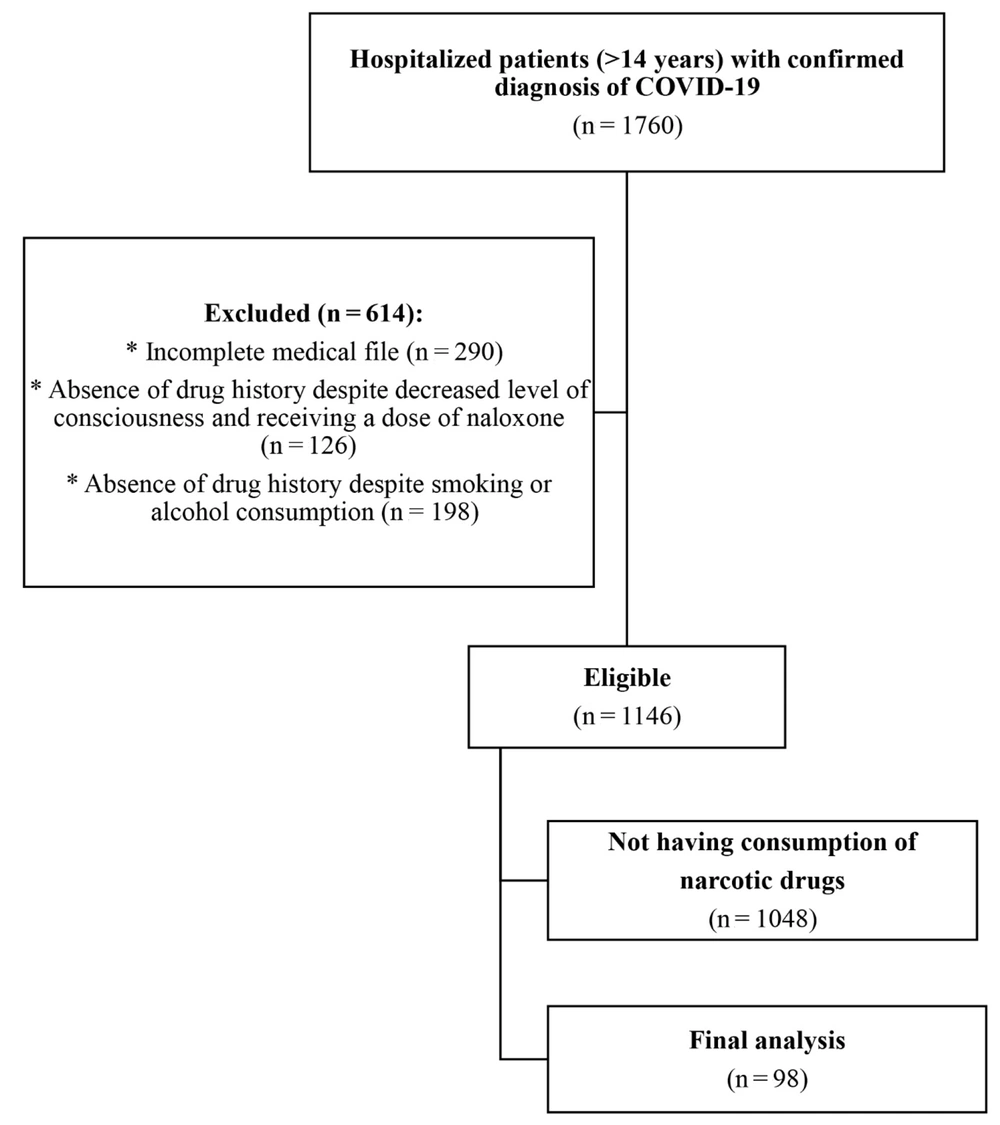
A total of 1,760 patients with a confirmed diagnosis of COVID-19 were hospitalized during the study period, and their medical files were reviewed. However, 614 patients were excluded from the study. Of the remaining 1,146 eligible patients, 98 (8.55%) reported opioid drug use and were included in the final analysis (Figure 1).
The mean ± SD age of the patients was 61.40 ± 14.50 years (range: 24 - 95), with the majority (43.9%) in the age group of 50 - 75 years. Eighty-one (82.7%) of the patients were male (P < 0.001), and the mean age was statistically similar between males (60.51 ± 15.46 years) and females (65.35 ± 8.32 years, P = 0.8). The overall mortality rate was 30.61%. Patients were divided into two groups: Survivors and non-survivors. Both groups were comparable in demographic characteristics, except for age, which was higher among non-survivors (P = 0.004).
As shown in Table 1, the most commonly used opioid substances were opium (61.2%) and methadone (54.1%). Notably, some patients used multiple drugs in combination, resulting in total percentages exceeding 100%. Among the patients, 27 (27.6%) used opioids by inhalation, 25 (25.5%) used them orally, and for 48 (46.9%), the method of use was not specified in their medical files. Opium use was significantly more frequent among survivors (P = 0.01), but the frequency of other opioid combinations was statistically similar between the two groups.
| Variables | Total (n = 98) | Non-survivors (n = 30) | Survivors (n = 68) | P-Value |
|---|---|---|---|---|
| Age | 61.40 ± 14.50 | 67.79 ± 12.60 | 58.50 ± 14.46 | 0.004 b (3.11, 15.48) |
| Gender | 0.26 | |||
| Male | 81 (81.6) | 27 (90) | 54 (79.4) | |
| Female | 17 (17.3) | 3 (10) | 14 (20.6) | |
| Comorbidity | ||||
| Total | 82 (83.7) | 25 (83.3) | 57 (83.8) | 0.99 |
| Chronic heart disease | 25 (25.5) | 7 (23.3) | 18 (26.5) | 0.81 |
| Diabetes | 28 (28.6) | 9 (30) | 19 (27.9) | 0.99 |
| Hypertension | 37 (37.8) | 15 (50) | 22 (32.4) | 0.12 |
| Chronic renal failure | 11 (11.5) | 6 (20) | 5 (7.4) | 0.09 |
| Liver diseases | 4 (4.1) | 0 (0) | 4 (5.9) | 0.31 |
| Cancers | 3 (3.1) | 1 (1.3) | 2 (2.9) | 0.99 |
| Asthma | 5 (5.1) | 0 (0) | 5 (7.4) | 1.18 |
| COPD | 3 (3.1) | 1 (1.3) | 2 (2.9) | 0.99 |
| Hypothyroidism | 3 (3.1) | 0 (0) | 3 (4.4) | 0.36 |
| Hyperthyroidism | 1 (1.0) | 0 (0) | 1 (1.5) | 0.99 |
| Psychiatric diseases | 9 (9.2) | 4 (13.3) | 5 (7.4) | 0.45 |
| Smoking | 69 (70.4) | 20 (87) | 49 (86) | 0.99 |
| Chronic alcohol consumption | 18 (18.4) | 5 (23.8) | 13 (32.5) | 0.56 |
| Type of opioid substance consumed | ||||
| Opium | 60 (62.1) | 24 (80) | 36 (52.9) | 0.01 b |
| Methadone | 53 (54.1) | 10 (33.3) | 35 (51.5) | 0.13 |
| Opium syrup | 35 (35.7) | 13 (43.3) | 22 (32.4) | 0.36 |
| Heroin | 21 (21.4) | 3 (10) | 18 (26.5) | 0.11 |
| Morphine | 1 (1.0) | 0 (0) | 1 (1.5) | 0.99 |
| Pethidine | 0 (0) | 0 (0) | 0 (0) | - |
| Buprenorphine | 0 (0) | 0 (0) | 0 (0) | - |
| Diphenoxylate | 0 (0) | 0 (0) | 0 (0) | - |
| Opium + methadone | 5 (0.5) | 7 (23.7) | 9 (13.2) | 0.24 |
| Opium + opium syrup | 16 (16.3) | 10 (33.3) | 21 (30.9) | 0.82 |
| Opium + opium syrup + methadone | 31 (31.6) | 3 (10) | 6 (8.8) | 0.99 |
| Heroin + methadone | 9 (9.2) | 2 (6.7) | 13 (19.1) | 0.14 |
| Others | 15 (15.3) | 2 (6.7) | 3 (4.4) | 0.64 |
Abbreviation: COPD, chronic obstructive pulmonary disease.
a Values are expressed as mean ± SD or No. (%).
b Statistically significant.
Both groups had similar vital signs at the time of admission, except for diastolic blood pressure (DBP) and peripheral oxygen saturation, which were lower in the non-survivor group (P = 0.04 for both). Additionally, the frequency of AVPU scores differed significantly between the groups (P < 0.001). The most common clinical symptoms at the time of admission were dyspnea (69.4%), chills (37.8%), myalgia (34.7%), and nonproductive cough (24.5%). All clinical symptoms were similar between the two groups, as shown in Table 2.
| Variables | Total (n = 98) | Non-survivors (n = 30) | Survivors (n = 68) | P-Value | 95% Confidence Interval |
|---|---|---|---|---|---|
| Transferred by EMS | 39 (39.8) | 7 (46.7) | 32 (71.1) | 0.12 | - |
| Vital signs at the time of admission | |||||
| Systolic blood pressure (mmHg) | 128.27 ± 21.26 | 122.09 ± 18.39 | 131.50 ± 22.13 | 0.85 | -1.35, 20.17 |
| DBP (mmHg) | 76.11 ± 11.77 | 71.91 ± 11.65 | 78.21 ± 11.39 | 0.04b | 0.31, 12.28 |
| Heart rate (per minute) | 92.76 ± 24.24 | 88.22 ± 21.11 | 94.80 ± 25.43 | 0.24 | -4.47, 17.72 |
| Respiratory rate (per minute) | 23.84 ± 10.93 | 27.64 ± 14.51 | 22.13 ± 8.46 | 0.07 | -11.49, 0.46 |
| Body temperature (°C) | 44.37 ± 49.71 | 37.07 ± 0.94 | 47.84 ± 6.22 | 0.35 | -11.93, 33.47 |
| O2 saturation (%) | 79.50 ± 17.29 | 73.56 ± 19.11 | 82.22 ± 15.83 | 0.04b | 017, 17.17 |
| AVPU | < 0.001b | ||||
| Alert | 49 (50) | 5 (16.7) | 44 (64.7) | - | - |
| Verbal response | 25 (25.5) | 1 (3.3) | 24 (35.3) | - | - |
| Painful response | 0 (0) | 0 (0) | 0 (0) | - | - |
| Unresponsive | 24 (24.5) | 24 (80) | 0 (0) | - | - |
| Clinical symptoms | |||||
| Dyspnea | 68 (69.4) | 23 (76.7) | 45 (66.2) | 0.35 | - |
| Chill | 37 (37.8) | 12 (40) | 25 (36.8) | 0.82 | - |
| Myalgia | 34 (34.7) | 10 (33.3) | 24 (35.3) | 0.99 | - |
| Non-productive cough | 24 (24.5) | 8 (26.7) | 26 (23.5) | 0.8 | - |
| Anorexia | 23 (23.5) | 9 (30) | 14 (20.6) | 0.44 | - |
| Weakness and fatigue | 21 (21.4) | 8 (26.7) | 13 (19.1) | 0.43 | - |
| Nausea and vomiting | 21 (21.4) | 10 (33.3) | 11 (16.2) | 0.7 | - |
| Productive cough | 20 (20.4) | 4 (13.3) | 16 (23.5) | 0.29 | - |
| Chest pain | 18 (18.4) | 5 (16.7) | 13 (19.1) | 0.79 | - |
| Headache | 18 (18.4) | 6 (20) | 12 (17.6) | 0.99 | - |
| Respiratory distress | 8 (8.2) | 2 (6.7) | 6 (8.8) | 0.99 | - |
| Rhinorrhea | 5 (5.1) | 1 (3.3) | 4 (5.9) | 0.99 | - |
| Taste disorder | 4 (4.1) | 2 (6.7) | 2 (2.9) | 0.58 | - |
| Odor Disorder | 2 (2) | 0 (0) | 2 (2.9) | 0.99 | - |
| Sore throat | 0 (0) | 0 (0) | 0 (0) | - | - |
Abbreviations: EMS, Emergency Medical Services; SD, standard deviation; DBP, diastolic blood pressure.
a Values are expressed as mean ± SD or No. (%).
b Statistically significant.
Moreover, laboratory findings at the time of admission were similar between the two groups, except for C-reactive protein (CRP), which was significantly higher in non-survivors (P< 0.001) (Table 3).
| Variables | Total (n = 98) | Non-survivors (n = 30) | Survivors (n = 68) | P-Value | 95% Confidence Interval |
|---|---|---|---|---|---|
| WBC (109/L) | 8.92 ± 3.94 | 9.25 ± 40.6 | 7.78 ± 3.90 | 0.60 | -2.21, 1.29 |
| Hemoglobin (g/dL) | 12.66 ± 2.67 | 12.59 ± 2.58 | 12.69 ± 2.72 | 0.86 | -1.80, 1.30 |
| BUN (mg/dL) | 28.35 ± 21.12 | 34.41 ± 25.94 | 25.68 ± 18.36 | 0.11 | -19.48, 2.11 |
| Creatinin (mg/dL) | 2.17 ± 2.68 | 1.75 ± 1.36 | 2.37 ± 3.11 | 0.30 | -0.55, 1.80 |
| Serum sodium (mEq/L) | 138.39 ± 4.75 | 137.47 ± 5.93 | 238.80 ± 40.8 | 0.27 | -10.7, 3.75 |
| Serum potassium (mEq/L) | 4.60 ± 0.86 | 4.74 ± 0.91 | 4.54 ± 0.85 | 0.31 | -0.59, 0.19 |
| Blood suger (mg/dL) | 170.66 ± 117.53 | 209.58 ± 176.66 | 154.84 ± 79.06 | 0.14 | 128.40, 18.94 |
| ALT (U/L) | 100.63 ± 241.08 | 67.0 ± 76.41 | 114.31 ± 281.42 | 0.42 | -69.07, 163.68 |
| AST (U/L) | 104.49 ± 230.71 | 108.96 ± 180.07 | 102.68 ± 248.02 | 0.91 | -118.09, 105.53 |
| LDH (U/L) | 909.53 ± 103.95 | 1006.35 ± 491.67 | 863.76 ± 10.2.66 | 0.56 | -633.01, 347.85 |
| CPK (mcg/L) | 368.73 ± 101.58 | 579.39 ± 174.58 | 271.82 ± 343.44 | 0.41 | -1067.59, 452.44 |
| ESR (mm/hr) | 48.0 ± 34.92 | 56.40 ± 33.82 | 44.99 ± 35.21 | 0.28 | -32.42, 9.61 |
| CRP (mg/L) | 62.0 ± 30.37 | 78.73 ± 12.78 | 55.12 ± 32.82 | < 0.001 a | -33.83, -13.39 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; CPK, creatine phosphokinase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; LDH, Lactate dehydrogenase; SD, standard deviation; WBC, white blood cell.
a Statistically significant.
The characteristics of patients' hospitalizations are presented in Table 4. Fourteen patients (14.3%) were admitted to the intensive care unit (ICU), with a significantly higher proportion among non-survivors (P = 0.001). Additionally, the length of ICU stay was longer for non-survivors (P = 0.03). However, the total duration of hospitalization was similar between the two groups (P = 0.43).
| Variables | Total (n = 98) | Non-survivors (n = 30) | Survivors (n = 68) | P-Value | 95% Confidence Interval |
|---|---|---|---|---|---|
| Length of hospital stay (day) | 7.85 ± 6.95 | 8.77 ± 7.62 | 7.47 ± 6.68 | 0.43 | -4.54, 1.93 |
| Length of ward admission (day) | 6.77 ± 6.25 | 6.27 ± 7.08 | 6.98 ± 5.91 | 0.63 | -2.21, 3.64 |
| ICU admission | 14 (14.3) | 10 (33.3) | 4 (5.9) | 0.001 b | - |
| Length of ICU admission (day) | 0.85 ± 2.71 | 1.83 ± 3.11 | 0.41 ± 2.41 | 0.03 b | -2.71, -0.14 |
Abbreviations: ICU, intensive care unit; SD: standard deviation.
a Values are expressed as mean ± SD or No. (%) unless otherwise indicated.
b Statistically significant
Univariate logistic regression analysis was performed to identify prognostic factors for in-hospital mortality among the patients. Variables with P < 0.2, including age, chronic renal disease, hypertension, DBP, peripheral oxygen saturation, respiration rate per minute, blood urea nitrogen (BUN), blood sugar, CRP, and ICU length of stay, were entered into the multivariate logistic regression model (Table 5).
| Variables | β | SE | OR | P-Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | -0.06 | 0.02 | 0.95 | 0.01 a | 0.91 | 0.99 |
| Hypertension (reference: Yes) | -0.74 | 0.45 | 0.48 | 0.01a | 0.20 | 1.15 |
| Chronic kidney disease (reference: Yes) | -1.15 | 0.65 | 0.32 | 0.08 a | 0.09 | 1.14 |
| Transferred by EMS | 1.03 | 0.61 | 2.81 | 0.9 | 0.85 | 9.36 |
| DBP | 0.05 | 0.03 | 1.06 | 0.05 a | 1.0 | 1.11 |
| O2 saturation | 0.03 | 0.1 | 1.03 | 0.04 a | 1.0 | 1.06 |
| Respiratory rate | -0.05 | 0.03 | 0.95 | 0.06 a | 0.91 | 1.0 |
| BUN | 0.02 | 0.01 | 0.98 | 0.08 a | 0.96 | 1.0 |
| Serum sodium | 0.06 | 0.05 | 1.06 | 0.2 | 0.97 | 1.17 |
| Blood sugar | -0.01 | 0.01 | 0.99 | 0.06 a | 0.99 | 1.0 |
| CRP | -0.04 | 0.02 | 0.96 | 0.01 a | 0.93 | 0.99 |
| ICU admission (reference: Yes) | -2.08 | 0.65 | 0.13 | 0.01 a | 0.04 | 0.44 |
Abbreviations: BUN, blood urea nitrogen; CI, confidence interval; CRP, C-reactive protein; EMS, emergency medical service; ICU, intensive care unit; OR, odd's ratio; SE, standard error; DBP, diastolic blood pressure.
a Statistically significant.
The results of the multivariate logistic regression model indicated that CRP (OR = 0.92, P = 0.02) was the only independent predictor of in-hospital mortality among hospitalized COVID-19 patients with a history of opioid substance use (Table 6).
| Variables | β | SE | OR | P-Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Respiratory rate | -0.16 | 0.11 | 0.85 | 0.13 | 0.98 | 1.05 |
| CRP | -0.09 | 0.04 | 0.92 | 0.02 a | 0.85 | 0.99 |
| ICU admission | -4.27 | 2.17 | 0.01 | 0.05 | 0.005 | 0.403 |
Abbreviations: CI, confidence interval; ICU, intensive care unit; OR, odd's ratio; SE, standard error.
a Statistically significant.
5. Discussion
This study aimed to evaluate the prognostic factor for in-hospital mortality in confirmed COVID-19 patients with a history of opioid combination consumption in southern Iran. In this study, the COVID-19-related mortality rate among patients with opioid combination consumption was 30.6%. In a comprehensive study on COVID-19 patients during the first wave of the epidemic in China, Deng et al. reported a case fatality risk of 5.65%, with the highest rate in Wuhan (7.71%) and the lowest in Hubei province (0.86%) (13). Ebrahimi et al.'s study (4) reported a mortality rate of 9.5% among re-infected COVID-19 patients. Similarly, Li et al., in a systematic review and meta-analysis assessing the epidemiology, clinical features, risk factors, and treatment outcomes in COVID-19 patients, reported an overall mortality rate of 5.6% (14). In Iran, mortality rates among COVID-19 patients were reported as 23% in a study by Homayounieh et al. (15) and 13.72% by Malekpour Alamdari et al. (16). A comparison of these studies with our findings suggests that COVID-19 mortality rates are higher among opioid combination users, providing no evidence of a protective effect of opioids in preventing or alleviating COVID-19.
During the study period, 98 patients with a confirmed diagnosis of COVID-19 had a history of opioid combination consumption, indicating a prevalence of 8.55%. In a study by Soori et al., the prevalence of opioid combination consumption among interurban public transportation drivers was reported as 14.1% (17). Similarly, Noori et al. found that 2.4% of 6,027 participants in a study on Tehran's population were addicted to opium (18), a rate lower than that observed in the present study. In a cross-sectional household survey by Roshanpajouh et al., which enrolled 6,024 residents of Tehran aged 15 - 64 years, the prevalence of opioid combination consumption was found to be 7.3% (19). These findings suggest that the prevalence of opioid combination consumption is slightly higher in patients with a confirmed diagnosis of COVID-19 compared to other populations in Iran, contradicting the hypothesis that addicts are less frequently infected with COVID-19.
According to the results of this study, 82.7% of the participants were male, with opium (61.2%) and methadone (54.1%) being the most frequently used substances. Additionally, 27.6% of the patients consumed opioid combinations by inhalation, and 25.5% used them orally. Furthermore, the findings showed that opium usage was significantly higher among non-survivors. However, the frequency of using other opioid drugs was comparable between survivors and non-survivors.
In line with our findings, Shahbazi et al. reviewed 2,957 death files submitted to the Iran National Forensic Medicine Department due to opioid combination abuse. They reported that most cases involved single, male, low-educated, and low-income individuals, with opium, glass, and heroin being the most common drugs, respectively (20). Similarly, another study investigated opioid combination abuse-related deaths in corpses referred to the Forensic Medicine Department of Khorasan province, Iran, and found that most fatalities involved males (93.4%), individuals with intermediate school education (27%), and self-employed persons (24.6%). The most lethal route of opioid combination consumption was injection, and opium was the most commonly used compound (32.7%) (20).
In another study, Shokrzadeh et al. reviewed 272 death files related to the use of morphine, opium, and other combinations referred to the Forensic Medicine Department of Mazandaran province, northern Iran, between 2007 and 2012. They found that most deaths (39.4%) were linked to opioid poisoning. The majority of these individuals were male, married, self-employed, had a diploma or lower education, and used injection (42.2%) as the primary method of drug intake (21). Additionally, Soori et al. reported that opium (55%), morphine (21.3%), and methadone (8.5%) were the most commonly used opioid combinations among drivers. A logistic regression model in the study revealed a statistically significant association with educational level (17).
The mean ± SD age of patients in this study was 61.40 ± 14.50 years, with the oldest subject aged 95 years and the youngest 24 years old. Most patients were within the 50 - 70 years age range (43.9%). The mean ± SD age of non-survivors was 67.79 ± 12.60 years, significantly higher than that of survivors. In Shokrzadeh et al.'s study, the mean ± SD age of non-survivors due to opioid abuse was 39.46 ± 14.27 years (21). Similarly, Hejazi et al. found that the highest percentage of deaths (11.4%) due to opioid combination abuse occurred in the 21 - 30 years age group (22). Shahbazi et al. reported an overall drug abuse mortality rate of 53.28 per million in Iran (20).
Regarding laboratory findings at the time of admission, survivors and non-survivors differed significantly only in the mean CRP value, which was higher in non-survivors. This study demonstrated that CRP was the only independent predictor of in-hospital mortality among opioid combination users with confirmed COVID-19, increasing the risk of mortality by 71 times. Consistent with these results, a systematic review and meta-analysis by Zhang et al. on 1,905 patients found a significant association between elevated CRP levels and increased disease severity and mortality in COVID-19 patients (23). Similarly, Wang et al. reported that patients with more severe disease exhibited significantly higher CRP levels, suggesting CRP as a valuable predictor of COVID-19 disease exacerbation (24).
This study had some limitations, including its short duration and single-center design. As a retrospective study focusing on opioid combination users, medical files lacking information about opioid use (due to denial or inaccurate self-reporting) were excluded. Consequently, the actual prevalence of opioid combination consumption may be higher than reported in this study. Future research is recommended on larger populations, across multiple centers, and with longer follow-up periods.
5.1. Conclusions
The results of this study revealed a mortality rate of 30.61% among COVID-19 patients with a history of opioid combination consumption. These findings suggest that opioid combination consumption does not play a role in preventing or alleviating COVID-19. Overall, the level of CRP emerged as the sole predictive factor for in-hospital mortality among confirmed COVID-19 patients with opioid substance consumption.