1. Context
Vibrio is a genus of frequently pathogenic autochthonous marine bacteria. Widely recognized toxigenic V. cholerae strains are uniquely associated with large scale waterborne outbreaks of epidemic cholera, a severe diarrheal disease. Less well known, but of greater concern in the United States are the non-cholerae species of Vibrio, 15% of which are known or suspected human pathogens capable of causing gastrointestinal illness, primarily sepsis, and soft tissue infection - together recognized as vibriosis (1). Species most commonly identified in such cases are V. vulnificus and V. parahaemolyticus, but include a number of other increasingly recognized organisms such as V. alginolyticus, V. fluvialis, V. mimicus and V. metschnikovii (2, 3). The focus of this scoping review is the complications and sequelae of infections from non-cholerae species of Vibrio.
Disease may present as mild to severe gastroenteritis usually with diarrhea, fever, and vomiting. Severe illness is commonly associated with hepatic comorbidities, diabetes, or other pre-existing conditions; however, otherwise healthy individuals may be severely affected (4). Recognized serious complications and sequelae for otherwise healthy individuals include sepsis and necrotizing fasciitis sometimes leading to amputation. Fatality rates vary by species with 29 - 35% of V. vulnificus infections and 1 - 1.4% of V. parahaemolyticus infections resulting in death respectively (5, 6).
Infection by Vibriospp. may occur through the consumption of contaminated food or water, particularly seafoods, or following cutaneous/parenteral exposure. All non-cholerae Vibrio spp. are estimated to have caused 34,600 domestically acquired waterborne illnesses in 2014 (5). Vibrio parahaemolyticus was estimated to have caused 20,800 of these waterborne illnesses, V. alginolyiticus 12,700, and V. vulnificus 188 (5). Scallan et al. estimated that non-cholerae Vibrio spp. caused 52,324 domestically acquired foodborne illnesses, with V. parahaemolyticus accounting for 34,664 of these illnesses, V. vulnificus 96, and other non-cholerae species accounting for 17,564 domestically acquired foodborne illnesses. While the number of infections is relatively low compared to other food and waterborne pathogens, with a high mortality rate and new data emerging on long-term sequelae, the burden of these infections is potentially significant and needs further exploration (6).
Infections caused by Vibrio spp. have historically been associated with tropical and subtropical waters, however global rises in sea temperatures due to climate change are expected to increase the natural range of these potential pathogens, and therefore the overall burden of disease in temperate coastal areas including many parts of the US (7, 8). Analysis of data from the US Cholera and Other Vibrio Illness Surveillance (COVIS) system has already identified increases in the incidence of vibriosis consistent with this pattern (9).
Estimates of the consequences of disease in addition to estimates of incidence, hospitalizations, and deaths are increasingly sought after for studies of disease burden and cost. In this paper, we provide an overview of the current state of the field with regard to complications and long-term sequelae associated with vibriosis, particularly those that may substantially affect the accuracy of burden and cost estimates. One question that motivated this review was gaining a better understanding of the extent of evidence regarding the common association between severe soft tissue outcomes, especially amputation. We have also sought to identify substantial knowledge gaps in the available literature, including routes of transmission that must be addressed to form the most accurate picture of the true impact of non-cholerae vibriosis.
2. Materials and Methods
A scoping review was conducted using the framework developed by Arksey and O’Malley and refined by Levac et al., to investigate complications and long-term outcomes of Vibrio spp. infections. Initial search criteria included the genus name and general terms for widely recognized outcomes of bacterial infection including death, necrotizing fasciitis, and septic shock (10, 11). Phrases indicating potential economic, and quality of life impacts were also included. Terms were expanded based on these initial results to form a maximally inclusive search strategy in consultation with a medical research librarian (JM). Final search terms used are available in Appendix 1 in Supplementary File. This search was adapted to the specific requirements of each of four widely recognized independent databases of scientific and/or medical literature: PubMed Central, Scopus, Web of Science, and Embase. The complete results from each search were compiled and duplicate studies removed.
DistillerSR was used to allow two independent reviewers to screen titles and abstracts based on central inclusion/exclusion criteria (Table 1); in cases of disagreement a third independent reviewer was utilized to determine appropriateness of inclusion. If it was not possible to fully evaluate criteria from the abstract alone, the study was included for further review. Included papers were subsequently removed based on the identification of exclusion criteria within the full text or included for further data extraction. Relevant data was extracted using a custom form created in Qualtrics, to allow subsequent data analysis. Fifteen percent of full text determinations and, where relevant, the associated completed data extraction forms, were chosen at random for quality control review by a second team member. In the initial screening, case studies of fewer than five individuals were excluded; however, it was later determined that these should be included due to the often-sporadic nature of non-cholera Vibrio infection and the potential for infrequently reported complications and long-term sequelae to be identified through these reports.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Publication date | Articles published between January 1, 2000, and May 26, 2020 | Articles published outside of this date range |
| Study design | Original research, case reports, case series | Review articles, conference proceedings, medical school curriculum guides |
| Pathogen | Non-cholera Vibrio spp. | Vibrio cholerae |
| Outcomes | Death, necrotizing fasciitis or similar tissue infection, amputation, abscess, organ failure, shock, sepsis, bacteremia, disseminated intravascular coagulation; any other long-term or chronic outcome of infection, i.e., irritable bowel syndrome, neurological complications, arthritis, etc. (See Appendix 1 in Supplementary File) | Uncomplicated acute infection, e.g., soft tissue, gastroenteritis; hospitalization, incidence, or mortality rates associated with acute infection only; disease burden or economic impact studies relating specifically to acute infection |
| Language | English | All other languages |
| Population | Human | Animal models, exclusively in-vitro or in-silico analyses |
3. Results
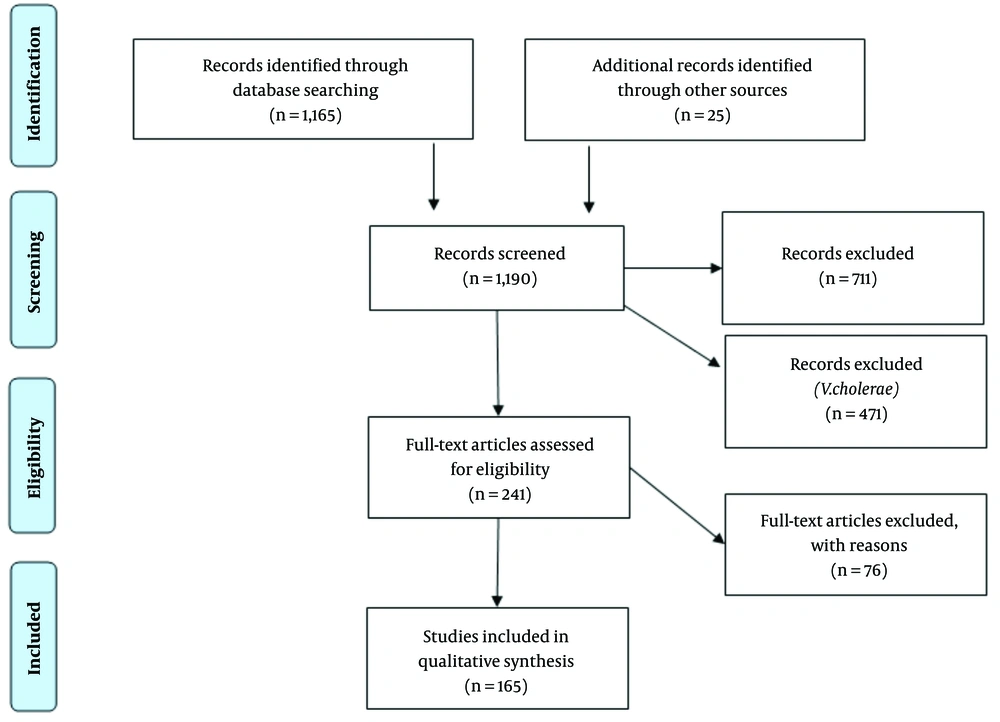
Ultimately, 1,170 non-duplicate studies were imported to DistillerSR, of which a further five were determined to be duplicates with minor inconsistencies in title, etc. From these, 479 studies were deemed relevant for full text review, based on initial screening criteria. Figure 1 includes individual case studies and series of fewer than five cases that were initially excluded. Because of the low frequency of less common, but potentially relevant complications and sequelae, individual case reports and small case series were reviewed specifically for disease outcomes and potential sequelae. A further 25 studies were added based on hand screening of references and relevant publications available after the initial search date in November 2018 to May 2020.
Of the total selected for full text screening, 241 appeared to potentially contain relevant data regarding non-cholerae Vibrio species. Seventy-six additional studies were excluded based on established exclusion criteria (Table 1). Data was extracted from 165 publications and from those, there were 90 individual case reports and 23 small case series.
The non-cholerae Vibrio species most commonly identified as the etiologic agent, by a wide margin, was V. vulnificus (137 studies), followed by V. parahaemolyticus (30 studies), V. alginolyticus (25 studies) and V. fluvialis (11 studies). Eight other species are individually described in at least one study while some infections were identified as mixed Vibrio spp. or identified only to the genus level (14 studies).
Necrotic lesions including necrotizing fasciitis (NF) were the most commonly reported outcome of infection by all species (102/165 total studies), followed by death (92/165), and sepsis or septic shock (90/165). Serious complications of the skin other than or in addition to NF, including cellulitis (10 studies), bullous lesions (13 studies), hemorrhage (3 studies) were reported by a total of sixteen publications. Amputation was reported as an outcome by 33 studies (including four case series of greater than 10 cases, five case series with 10 or fewer cases, and five single case studies), single or multi-system organ failure was reported by 28 reports (including four case series of greater than 10 cases, five case series with 10 or fewer cases, and 13 single case studies), and hematologic complications, particularly disseminated intravascular coagulation, were specifically addressed in 10 reports (including two case series of greater than 10 cases, two case series with 10 or fewer cases, and five single case studies). Other consistently reported complications included compartment syndrome (five total studies including two single cases), spontaneous peritonitis (six total studies including four single cases), skin grafts (four total studies including one single case), or severe respiratory complications, though the specific terminology varied and included respiratory distress, respiratory failure, pleural effusion, and pneumothorax (nine total studies including four single cases) (Appendices 2 and 3 in Supplementary File).
Rare but potentially significant outcomes reported in single publications (n = 1 for each), commonly case studies and small case series, included prolonged endophthalmitis, corneal ulcer, keratitis with reduced vision, or loss of vision; aortic aneurysm, non-thrombotic myocardial damage, cardiac arrythmia, endocarditis, aplastic anemia, intrahepatic duct stones, intussusception, hysterectomy, osteomyelitis, tendinitis, rhabdomyolysis, lesions of the basal ganglia, mental status deterioration, prolonged pain, or wound healing necessitating repeated debridement (n = 2), empyema (n = 2), and septic arthritis (n = 2) (additional detail available in Appendices 2, 3).
Of the studies reporting amputation as an outcome, V. vulnificus was the most commonly identified species associated with this specific outcome (Table 2). Of 33 studies reporting amputations, specific outcomes were reported for 192 individuals. Of these, 163 (84.9%) were associated with V. vulnificus, as compared to 11 (5.7%) amputations linked to V. alginolyticus, 8 (4.1%) linked to V. parahaemolyticus, 1 (0.5%) linked to V. fluvialis and 7 (3.6%) of amputations described as other Vibrio or not linked to a specific species. One study did not provide the total number of amputations but did describe 83% of those identified as linked to V. vulnificus. In most cases, the specific body site affected by the amputation was not given (138, 71.8%). Among the 54 cases for whom body site was reported, 30 (55.5%) affected the leg(s), 13 (24%) the arm(s), 8 (14.8%) hands, and 1 foot (1.8%). Only 23 cases were described as affecting the left side (13, 56.5%), right side (7, 30.4%) or as being bilateral in nature (3, 13%) (Table 2).
| Study; First Author (y) | Study Design | Exposure/Route of Infection | Total Amputation Cases | Body Sites/Locations | Vibrio species |
|---|---|---|---|---|---|
| Huang 2008 (12) | Retrospective cohort | Unknown (2), water (2), fish fin (3) | 7 | R upper extremity (5) L upper extremity (2) | V. vulnificus (5) V. parahemolyticus (1) other (1) |
| Kiratisin 2012 (13) | Retrospective cohort | Unknown | 1 | Bilateral BK | V. vulnificus |
| Mirron 2003 (14) | Case report/series | Water | 1 | R leg AK | V. vulnificus |
| Ralph 2007 (15) | Case report/series | Fish processing | 2 | L leg BK, L foot | V. vulnificus |
| Zaidenstein 2008 (16) | Chart review | Multiple | 9 | Leg (2), arm (1), hand (6) | V. vulnificus |
| Tsai 2011 (17) | Retrospective cohort | Wound | 7 | Arm AE (2), leg AK (2), leg BK (1), hand (1) | V. vulnificus |
| Hau 2011 (18) | Case report/series | Wound | 1 | L leg AK | V. vulnificus |
| Tang 2001 (19) | Retrospective cohort | Wound | 3 | R leg AK (1), R leg BK (1), L hand (1) | V. vulnificus |
| Fukui 2018 (20) | Case report/series | Water | 1 | R leg AK | V. vulnificus |
| H’ng 2005 (21) | Case series | Water (1), food (1) | 2 | R leg BK (2) | V. vulnificus |
| Lee 2014 (22) | Retrospective cohort | Multiple | 8 | No data | V. vulnificus |
| Kuo 2013 (23) | Retrospective cohort | Water | 18 | No data | V. vulnificus |
| Lee 2016 (24) | Retrospective cohort | Unknown (2), food (1) | 3 | No data | V. vulnificus |
| Koh 2018 (25) | Retrospective cohort | Wound | 1 | L arm AE | V. vulnificus |
| Chao 2012 (26) | Retrospective cohort | Multiple | 8 | No data | V. vulnificus |
| Hong 2014 (27) | Retrospective cohort | Multiple | 2 | No data | V. vulnificus |
| Ruppert 2004 (28) | Case series | Wound | 2 | R leg AK (1), L leg AK (1) | V. vulnificus |
| Slifka 2017 (29) | Case series | Multiple | 8 | No data | V. alginolyticus |
| Hsueh 2004 (30) | Retrospective cohort | Multiple | 6 | No data | V. vulnificus |
| Chen 2002 (31) | Case series | Food | 1 | Leg | V. vulnificus |
| Lu 2008 (32) | Case report | Unknown | 1 | L leg AK | V. vulnificus |
| Uchiyama 2007 (33) | Case report | Food | 1 | R leg AK | V. vulnificus |
| Mouzopoulos 2008 (34) | Case report/series | Wound | 3 | Leg AK (3) | V. vulnificus |
| Kuo 2007 (35) | Retrospective cohort | Water | 3 | Leg AK (3) | V. vulnificus |
| Tsai 2009 (36) | Retrospective cohort | Multiple | 6 | Leg AK (4), arm AE (2) | V. vulnificus |
| Dechet 2008 (37) | Disease Burden | Multiple | 44 | No data | V. vulnificus (30), V. parahemolyticus (5), V. alginolyticus (3), other (6) |
| Tsai-Nung 2019 (36) | Retrospective cohort | Multiple | 12 | No data | V. vulnificus |
| Tsao 2013 (38) | Retrospective cohort | Wound (5), non-wound (4) | 18 | No data | V. vulnificus |
| Chen 2012 (39) | Retrospective cohort | Multiple | 10 | No data | V. vulnificus |
| Guang-Liang 2012 (40) | Retrospective cohort | Multiple | 2 | No data | V. vulnificus |
| Yoder 2008 (41) | Disease Burden | Multiple | NA (percentages only) | No data | V. vulnificus (83%), other |
| Dziuban 2006 (42) | Disease Burden | Water | 6 | No data | V. vulnificus (5), V. parahemolyticus (1) |
| Tsai 2004 (43) | Retrospective cohort | Multiple | 4 | No data | V. vulnificus (2), V. parahemolyticus (1), V. fluvialis (1) |
Abbreviations: R, right; L, left; AK, above knee; BK, below knee; AE, above elbow.
Note: In cases where multiple species and body site are given, species was not linked to the site of infection. The level of detail reported for body site reflects the level of detail given in the original publication. Exposure source “Water” indicates exposure to salt water without an identified injury to the exposed skin, “Multiple” exposure indicates that the route of exposure was not linked directly to case outcomes.
The source of infection was infrequently reported in a way that allowed direct linkage to the outcome of infection, because even where total case numbers were provided by route of exposure, the outcomes for these groups were not clearly delineated. Cases may have been identified as unknown if the case did not recall a specific probable exposure. For amputations, of 192 individual cases only 57 patients (29.6%) could be associated with a specific route of exposure (Table 2). Of these identified exposures, 26 (43.8%) were described as wound-related, generally classified as such if the case reported a history of broken skin that did or could have come into contact with fish, shellfish, or the marine environment. Illnesses were commonly identified as foodborne only if the case reported recent consumption of raw or undercooked seafood (6/57, 10.5%). The second most common route of exposure was contact with water (43.9%); however, it is generally not known whether this indicates a waterborne or cutaneous exposure (Table 2).
The majority of studies addressed the presence of at least one known or suspected risk factor for serious disease in the study population or individual: 72.7% (120/165) reported some information on cases’ health status prior to contracting vibriosis. Specific risk factors examined differed widely between studies and were not reported in any consistent manner. Many (n = 37) studies examined only a single risk factor (e.g., asthma, dialysis) while others specifically noted the frequency of multiple possible risk factors for the case patients studied or reported outcomes for mixed risk factors. The most commonly included risk factors explicitly described were forms of hepatic disease (n = 49), renal disease (n = 15), diabetes (n = 25), cancers (n = 9) and immune disorders (n = 5). Cases were more commonly male (50 - 100% male in studies with 10 or more participants) and of advanced age (studies of 10 or more participants reporting average age ranged from 49 - 67.5 years, those reporting median age ranged from 49 - 73). Reporting appeared to reflect, unsurprisingly, higher total case reports from regions in which vibriosis is reportable, while severe illness was commonly reported from tropical and subtropical regions where these species would be expected to be environmentally present in higher numbers (Appendices 2 and 3 in Supplementary File).
4. Conclusions
Through this review we were able to identify several potentially important sequelae of vibriosis in addition to those most commonly recognized. However, we were able to draw only very limited conclusions regarding the importance or potential economic impact of these outcomes for several reasons. Long-term studies of vibriosis cases post-recovery are extremely uncommon, and some species remain entirely uninvestigated. Though serious or fatal disease has been noted in previously healthy individuals, most experience mild symptoms including classical gastroenteritis, leading to low healthcare utilization rates and subsequently low reporting. One particularly important indication of this work is that there is support for including previously uncounted costs associated with necrotizing fasciitis and amputation specifically in burden estimates for foodborne disease (44).
Commonly recognized presentations of vibriosis are generally associated with the route of infection: Gastroenteritis and primary septicemia resulting from ingestion of the bacterium, and wound infection that may be followed by secondary septicemia resulting from injury (2, 17, 23, 38). There is strong evidence that ingestion can and does result in a presentation involving soft tissue damage, necrotizing fasciitis, and amputation. Approximately ten percent of amputation cases identified in this review were directly attributed to consumption of food, generally raw seafood.
Frequently, the route of exposure was unknown or not recorded for specific outcomes, further weakening associations between disease presentation and route of exposure. Grouping of cases by general exposure to water obscures the true rate of food/waterborne oral ingestion illnesses as compared to cutaneous but non-wound associated cases. As representative examples, in a study of 93 hospitalized V. vulnificus patients, five recalled having consumed raw or undercooked seafood, 34 had exposure to a marine environment and the route of exposure for the remaining 54 was unknown (45). A study of reported V. vulnificus cases from 20 Japanese hospitals over the period 1984 - 2008 identified 37 cases, 82% of which were associated with foodborne exposure despite 97% presenting with skin lesions normally considered characteristic of cutaneous exposure (the remaining cases had an unknown route of exposure) (46). More than a third of these cases also presented with diarrhea, though identified GI symptoms were more frequently associated with nonfatal cases. Additionally, numerous case descriptions have linked foodborne exposure and mild gastroenteritis to progressive disease including necrotic lesions of previously uninjured limbs (31, 47-50). This review of the literature indicates that disease presentation should not be assumed to indicate the route of exposure; it is likely ingestion of non-cholerae Vibrio spp. may manifest as complex tissue infection requiring amputation. This link is clearest for V. vulnificus, largely because it is associated with more than 80% of known vibriosis-associated amputations, as is consistent with previous estimates (51).
It is possible that multiple significant outcomes remain unidentified or have, to date, been recognized only in a small number of cases such as those identified here due to the paucity of data for post-acute outcomes. Such atypical or underrecognized complications and sequelae are often severe. Known but relatively infrequent reported effects include severe respiratory complications and disseminated intravascular coagulation, while rare outcomes as varied as vision loss, septic arthritis, and myocardial damage have been identified in case reports as potentially associated with vibriosis and, if established by further study as true sequelae, could substantially alter cost models based on the long term need for care or a change in ability to work following recovery from an initial hospitalization.
Some key case studies appear to indicate potentially substantial underestimation of the burden of non-cholerae vibriosis and highlight the lack of information available regarding chronic sequelae. A single specialty clinic for hand surgery in Haifa, Israel reported five cases of hand injury resulting from the handling of a single marine species, carp, in the two-year period from 1999 - 2000. In four of the five cases, V. vulnificus was identified as the etiologic agent. Though there were no fatalities, one of the five suffered a life-threatening systemic infection and four of the five were left with long-term or permanent reduction in hand function. This indicates one area of known chronic outcomes associated with other foodborne pathogens, arthritic reactions and movement limitations, in which long-term disability information is almost entirely lacking for vibriosis.
We did not identify any long-term studies that sought to identify several of the potential sequelae known to exacerbate costs of other known Gram-negative bacterial foodborne pathogens, such as reactive arthritis and irritable bowel syndrome (IBS). Though it is possible that these are simply not true sequelae of vibriosis, V. cholerae has only recently been linked to IBS. At least this specific potential link should be further investigated for vibriosis, including less severe episodes of acute gastroenteritis (52, 53). These gaps in the current literature make it difficult to ascertain whether a particular outcome is not associated with vibriosis, whether it has not been detected because of the relative infrequency of vibriosis cases as compared to other food and waterborne pathogens, or if these potential connections simply have not been studied.
4.1. Conclusions
An accurate understanding of the long-term sequelae of non-cholerae vibriosis is crucial to the formulation of disease burden models used to inform research and public health priorities. Current models take into account costs of acute disease, including hospitalization and mortality, however we have determined that there is a paucity of data regarding possible complications and sequelae that is likely to lead to an underestimation of the true costs of vibriosis, even as it becomes increasingly relevant domestically through the expansion of its range due to climate change. Further studies, particularly long-term case-control and prospective cohort studies, should ideally be conducted to give further insight into currently unlinked but potentially economically important sequelae. Though we have been largely unable to identify sufficiently strong links to previously unrecognized post-infectious sequelae of vibriosis to merit their inclusion in current disease burden models, we have discovered substantial support for the inclusion of severe soft tissue outcomes including amputation in models of foodborne illness. These outcomes have not been associated with gastrointestinal disease and exposure via ingestion, and were, to date commonly assumed to be a direct result of parenteral exposure but are likely to be of great importance due to their permanent impact on function.