1. Background
Alzheimer’s disease (AD) is the prevailing type of neurodegenerative disease in older individuals (1). Clinically, AD is characterized by cognitive dysfunction, memory loss, and personality/behavioral changes. There are pathologically characterized by intracellular neurofibrillary tangles and extracellular β-amyloid (Aβ) plaques in the brain (2). Internal agents contributing to AD include genetics, aging, and other agents that are mainly inherited and cannot be modulated. Besides, external environmental agents effective on the risk of AD consist of chronic exposure to physical, chemical, and psychosocial hazards, as well as lifestyle agents (3).
Several researchers have presented persuasive evidence regarding bidirectional communication between the gut and the central nervous system (CNS). They revealed that the gut is involved in regulating brain functions, mainly through its microbiota and metabolic activity. Besides, they postulated that regulation of the gut ̓s natural flora could be a promising therapeutic strategy for neurodegenerative diseases. The natural residents of the gut include the majority of human microflora, constituting at least 1000 diverse bacterial populations. Studies have indicated that the human intestinal microbiota is primarily composed of Firmicutes and Bacteroidetes, which are the predominant phyla present. Various commensal microflora is exposed to dynamic alterations throughout life, as the number of species and the abundance of gut microbiome decrease remarkably with age (4). Uncommon alterations in the profile of the gut microbiome, known as dysbiosis, have a direct association with the pathophysiology of diseases affecting several far organs. The term “microbiota-gut-brain axis” represents the key role of the gut microbiome in the adjustment of brain functions. On the other hand, this axis provides a bidirectional connection by signaling between the gut microbiome and the brain via metabolic, endocrine, neural, and immune pathways, which are pivotal for consistent equilibrium in the brain (5). Therefore, it can be assumed that the gut microbiome has a potential association with the pathogenesis of AD and that a reduction in the gut microbiota variety can cause various pathologies in the brain, including inflammation, cerebrovascular degeneration, and Aβ aggregation (6).
The potential links between AD and the gut microbiota have attracted the researchers’ attention, and the microbiome has been proposed as a potential therapeutic target for AD. Recent APP/PS1 transgenic mouse models and human surveys have descovered that dysbiosis of the gut microbiota is correlated with the development of AD (7). In this regard, a recent animal study by Park et al. showed a significant difference at the phylum level, involving an increment in the Firmicutes population and a decline in Proteobacteria and Bacteroidetes populations in TgAPP/PS1 mice compared to wild-type mice (8). There was also a significant difference at the genus level, including an increase in the populations of Aerococcus, Jeotgalicoccus, Blautia, Pseudomonas, Clostridium, and Ruminococcus, and a reduction in the number of Lactobacillus and Corynebacterium species in TgAPP/PS1 mice.
2. Objectives
The current investigation was focused on assessing the abundance and composition of the gut microbiome populations of Enterobacter, Clostridium, Lactobacillus, and Bifidobacterium in a mouse model of AD and determining if there are any significant differences in these key gut bacteria levels between AD model mice and healthy control mice.
3. Methods
3.1. Alzheimer’s Disease Model
According to a survey by Hour et al., (9), sixteen young BALB/c mice (8 - 10 weeks), with a mean weight of 35 ± 2 g, were prepared from Iran University of Medical Sciences (Tehran, Iran) and accomodated in a controlled room in a 12:12 h light/dark cycle at a temperature of 23 ± 3°C and humidity of 50% ± 10%. All measures were performed in accordance with the International Guideline for Laboratory Animals. The mice were randomly allocated into two groups (eight mice per group): The AD model group receiving an intracerebroventricular (ICV) injection of streptozotocin (STZ) (3 mg/kg) and the control group administered with a phosphate-buffered saline (PBS) vehicle.
To anesthetize the mice, an intraperitoneal (IP) injection of 20 mg/kg of xylazine (Alfasan, the Netherlands) and 100 mg/kg of ketamine (Rotexmedica, Trittau, Germany) was administrated. The head of mice was then fixed in a stereotaxic device after shaving their hair, and their skulls were uncovered with a vertical incision along the sagittal line in the posterior part of the scalp. According to the Paxinos atlas of the mouse brain, a Hamilton microsyringe was used to administer STZ into the dorsal hippocampus bilaterally for three minutes at 3.6 mm posterior to the bregma, ± 2 mm lateral to the bregma, and 3.2 mm ventral to the skull surface. The needle stayed in its place for another two minutes after the injection. It was then slowly removed, and the scalp was sutured.
This study was conducted ethically, following all relevant guidelines and regulations for animal research. All animal procedures were approved (IR.IAU.SRB.REC.1399.109) by the ethical committee of Islamic Azad University, Science and Research Branch.
3.2. Behavioral Evaluations
After 16 to 17 days, the animals were assessed in terms of their learning and memory capacity using the Morris water maze (MWM) and passive avoidance response tests.
3.2.1. Morris Water Maze Test
As previously described (10), the spatial cognition of mice were assessed via the MWM task. Briefly, the equipment used in the MWM task consisted of a round water reservoir, segmented into four fictional quadrants with a platform (150 cm in diameter, 50 cm in depth) and containing water (approximately 22°C) up to 20 cm beneath the edge. The MWM test was conducted over six days, including the habituation day, acquisition phase, and probe trial stage. In the probe trial stage, the distance covered in the target quadrant and the time spent in this quadrant were assessed as two indicators of spatial memory. In both the learning phase and the probe trial stage, the locomotion of animals in water was captured by a camera situated atop the midpoint of the water reservoir; data were gathered by a computer using a water maze software program for behavioral assessment.
3.3. Passive Avoidance Response Test
According to the procedure described by Ader et al., a dual- chamber shuttle box with an interconnecting guillotine door was used for passive avoidance training (11). Initially, all mice were placed in the apparatus to become familiar with the environment. Next, they were led separately into the light compartment for ten seconds. The installed entrance of the dark compartment was then opened, and any animal that did not enter it within 60 seconds was eliminated from the research. The animal’s feet were subjected to electrical stimulation (0.5 mA, 50 Hz, 2 sec) after entering the dark chamber. On the following day, the mice without any foot shock were re-entered into the illuminated chamber. The delay in entering the dark compartment, referred to as the step-through latency (STL), as well as the overall duration spent in the dark chamber, was recorded. The time limit for the animal’s presence in the dark compartment and STL were 600 and 300 seconds, respectively. The animal’s avoidance of entering the dark chamber for up to 300 seconds was considered a successful passive avoidance response.
3.4. Nissl Staining
To distinguish the basic structure of healthy neurons from damaged ones in the spinal cord and the brain, cresyl violet (Nissl) staining is normally performed (12). After sample preparation and fixation, the sections were stained with Nissl stain. Next, images were acquired from the stained slides using an optical microscope (Carl Zeiss, Oberkochen, Germany). The loss of Nissl body uniformity, cell shrinkage, nucleolar pycnotization, and cytoplasmic and nucleus densities was finally determined, and the mean values were measured.
3.5. Investigating the Prevalence of Target gut Microbiota in Alzheimer’s Disease Mouse Model
3.5.1. DNA Extraction from Stool Samples
According to the protocol provided by the QIAamp® DNA Stool Mini Kit manufacturer (Qiagen Retsch GmbH, Hannover, Germany), whole microbial DNA was extracted from all stool specimens. The extracted DNA was immediately stored at -20°C. The quantity and quality of DNA samples were ascertained by a NanoDrop spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA) at 260 and 260/280 nm, respectively.
3.5.2. Primers and Probes
Table 1 presents the TaqMan probes and the specific sequences of primers against the selected species- or group-specific target sequences. To verify the specificity of primer pairs, the sequences were submitted to the FASTA database search program (http://www.ncbi.nlm.nih.gov) and ProbeMatch program (http://www.rdp.cme.msu.edu). The primer pairs were fabricated commercially by Pishgam Biotech Co. (Tehran, Iran).
| Organisms and Primer/Probe | Oligonucleotide Sequence | Product Size (bp) | Ref. |
|---|---|---|---|
| Bifidobacterium | 87 | (13) | |
| Primer-F | AAGCGATGGACTTTCACACC | ||
| Primer-R | TACGTAGGGTGCAAGCGTTA | ||
| Probe | CGCGACGAACCGCCTACGAGC | ||
| Bacteroides | 100 | (13) | |
| Primer-F | GTATGTCRCAAGCGTTATCC | ||
| Primer-R | AACGCAATACRGAGTTGAGC | ||
| Probe | TAGACGCGCTTTACGCCCAAT | ||
| Clostridium | 134 | (13) | |
| Primer-F | CGAACAGGATTAGATACCC | ||
| Primer-R | CTTTGAGTTTCACCGTTG | ||
| Probe | AAACGATGGATGCCCGC | ||
| Lactobacillus | 204 | (14) | |
| Primer-F | GTCTGATGTGAAAGCCYTCG | ||
| Primer-R | CCAGGGTATCTAATCCTGTTYG | ||
| Probe | YCACCGCTACACATGRAGTTCCACT |
3.5.3. Real-time Polymerase Chain Reaction Assay
To characterize bacterial DNA in the stool samples, a real-time TaqMan qPCR assay was conducted. The assay involved using 0.5 mL of forward primer, 0.5 mL of reverse primer, 0.5 mL of TaqMan probe, 12 mL of Probe Ex Taq (probe qPCR) master mix (Takara Bio Inc., Shiga, Japan), 1 mL of template DNA, and nuclease-free water to reach the final volume (20 mL). Amplification was carried out using a Gene Atlas 322 system (ASTEC, Japan) at 95°C for 30 seconds, followed by 40 cycles of denaturation at 95°C for five seconds and annealing/extension at different temperatures for 30 seconds for all bacteria. The mean values of triplicate samples were calculated for DNA analysis by real‐time PCR assay. To ensure quality and accuracy, Bifidobacterium (ATCC 27536), Lactobacillus (ATCC 11146), Clostridium (ATCC 25772), and Enterobacter (ATCC 21754) were used as controls (13). All bacterial strains were grown on brain-heart infusion (BHI) agar (Merck, Germany) to plot standard curves. Based on the Applied Biosystems tutorials, the standard curves were plotted and adjusted with the 16S rRNA gene copy number for each species (15).
3.6. Statistical Analysis
The collected data were statistically analyzed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Mean and SD were used to show descriptive results. To indicate the results regarding inferential statistics, independent t-test and if required Man-Whitney test were applied. P-values less than 0.05 were considered statistically significant.
4. Results
4.1. Behavioral Assessments
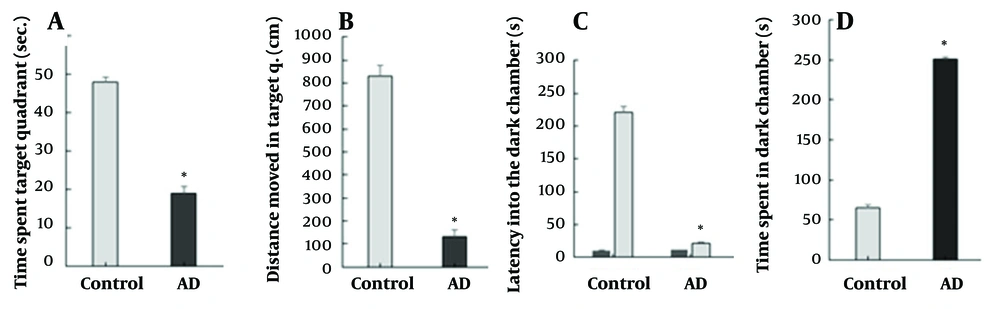
To assess the effects of STZ in the mouse model, the passive avoidance and MWM tests were carried out. The findings of the MWM test are presented in Figure 1A and B, indicating the duration and the displacement in the target quadrant, respectively. The STZ-treated mice showed a significant increase in the displacement and the duration in the target quadrant compared to the healthy mice (P ≤ 0.05) (Figure 1B). Before the electric shock application, the initial latency (IL) in entering the dark chamber was very short in the passive avoidance test and similar between the groups (Figure 1C). Nonetheless, the STL was shorter in the AD group compared to the control group (P ≤ 0.05) after the electric shock. The AD group exhibited a significantly longer duration spent in the dark chamber compared to the control group (with a significance level of P ≤ 0.05), as illustrated in Figure 1D. All descriptive results including mean and SD indicated in Figure 1A-D.
(A - D) behavioral analysis. MWM test, A, time spent in the target quadrant; B, distance moved in the target quadrant. Passive avoidance response test, C, latency into the dark chamber before and after applying the electric shock; D, time spent in a dark chamber. Error bars represent standard deviation; asterisks (*) represent significant P-value (P < 0.05) of group in comparison with control one.
4.2. Nissl-Stained Cells
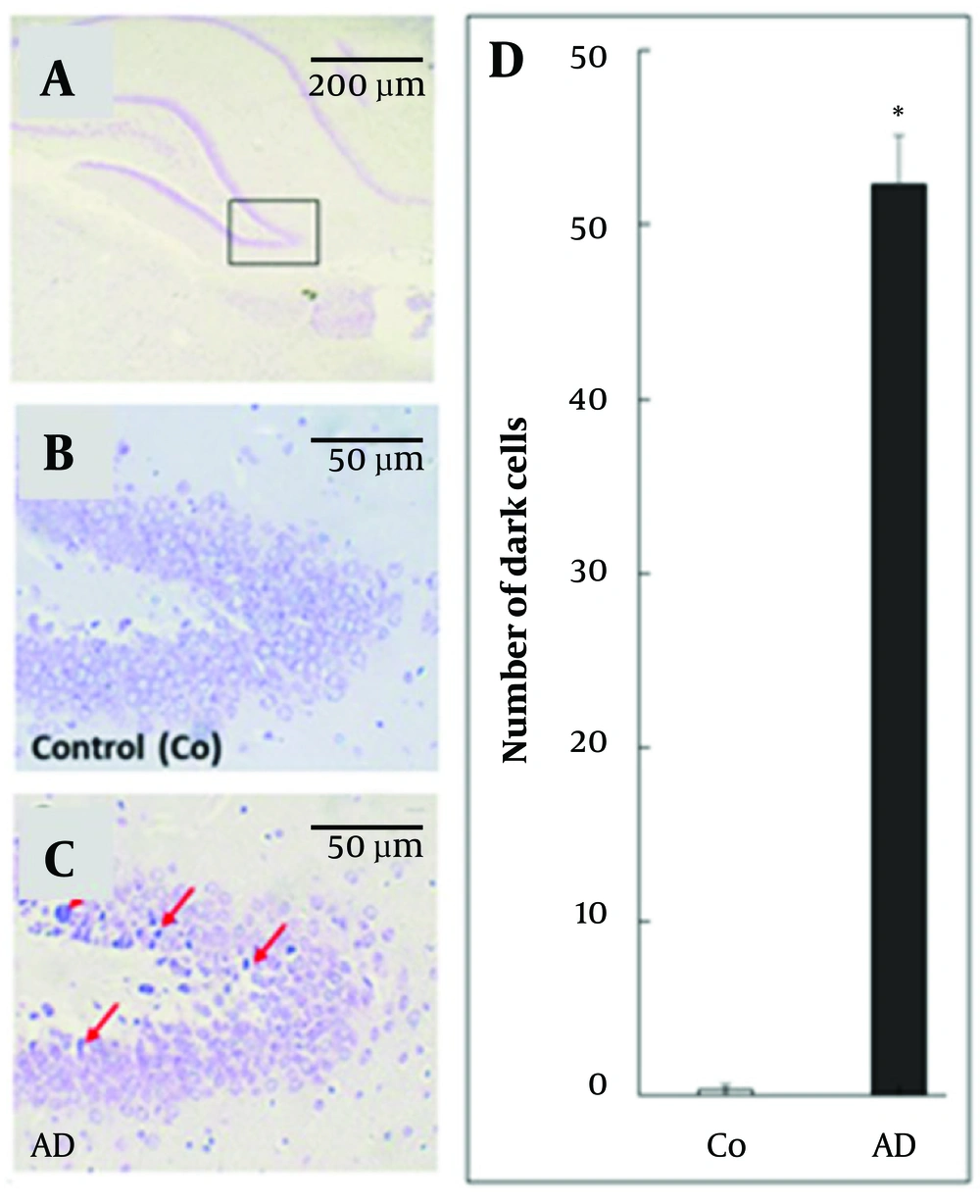
Ten weeks after cell treatment, the results of Nissl staining (Figure 2A-C) indicated that the control group had a noticeably lower quantity of dark cells compared to the AD group. Quantitative analysis (Figure 2D) of Nissl-stained images exhibited that the quantity of dark cells was significantly higher in the AD group compared to the control group (P ≤ 0.05). Descriptive results including mean and SD indicated in Figure 2D.
(A - D) Nissl staining of hippocampus in different mice groups. A, B, and C, red arrows show the dead or dark cell; D, quantitative analysis of dark cells. Error bars represent standard deviation; asterisks (*) represent significant P-value (P < 0.05) of group in comparison with control one.
4.3. Real-time Polymerase Chain Reaction Assay
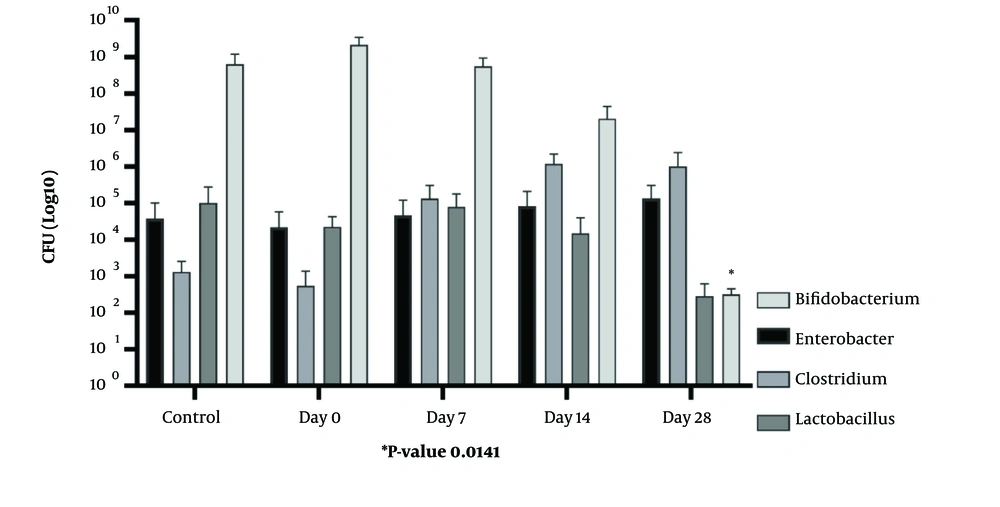
In this case-control study, a qPCR analysis was conducted to examine variations in the fecal microbiota combination, including Enterobacter, Clostridium, Lactobacillus, and Bifidobacterium populations, between the AD and control groups. Following the removal of any data outliers, an intra-group analysis was performed. This analysis revealed notable disparities in the abundance of the targeted bacterial species between the AD group and the healthy control group. The quantification results of bacterial genera indicated that the number of Lactobacillus, Enterobacter, and Clostridium bacteria was none-significantly higher in the AD group compared to the healthy group (P > 0.05), While, the Bifidobacterium population was significantly lower in the gut microbiota of the AD group compared to the healthy group (P < 0.05). Mean difference of Bifidobacterium CFU between AD group in day 28 and control group was -5.680 (95% CI: -11.3826 to -0.0174). All mean and SD of the intestinal bacterial genera between the study groups are presented in Table 2 and Figure 3. Also, all mean difference with 95% CI was presented in Table 3.
| Group | Model Group | Control | |||
|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 28 | ||
| Enterobacter | 3.34 ± 3.46 | 4.66 ± 4.78 | 4.92 ± 5.02 | 5.13 ± 5.15 | 4.58 ± 4.7 |
| Clostridium | 5.02 ± 5.08 | 5.13 ± 5.15 | 5.9 ± 6 | 6 ± 6.07 | 3.12 ± 3.02 |
| Lactobacillus | 2.74 ± 2.84 | 2.46 ± 2.43 | 5.18 ± 5.31 | 4.9 ± 4.9 | 5 ± 5.15 |
| Bifidobacterium | 9.33 ± 9.02 | 9.05 ± 8.77 | 7.44 ± 7.5 | 3.12 ± 2.15 | 8.8 ± 8.66 |
a Values are presented as mean ± SD.
| Group | Model Group (Mean Difference with Control Group- 95% CI) | |||
|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 28 | |
| Enterobacter | -1.240 (-5.6656 to 3.1856) | 0.080 (-5.0033 to 5.1633) | 0.340 (-4.8747 to 5.5547) | 0.550 (-4.7370 to 5.8370) |
| Clostridium | 1.900 (-2.5814 to 6.3814) | 2.010 (-2.5172 to 6.5372) | 2.780 (-2.3136 to 7.8736) | 2.880 (-2.2611 to 8.0211) |
| Lactobacillus | -2.260 (-6.7197 to 2.1997) | -2.540 (-6.8581 to 1.7781) | 0.180 (-5.4293 to 5.7893) | -0.100 (-5.4904 to 5.2904) |
| Bifidobacterium | 0.530 (-8.9519 to 10.0119) | 0.250 (-9.0961 to 9.5961) | -1.360 (-10.0472 to 7.3272) | -5.680 (-11.3826 to -0.0174) a |
a Represent significant P-value (P < 0.05) of group in comparison with control one. Other groups didn't show significant P-value (P > 0.05).
5. Discussion
Several studies have reported correlations between an imbalanced microbial community and gastrointestinal tract diseases (e.g., inflammatory bowel disease) and CNS disorders (e.g., AD) (16). Accordingly, in the current investigation, it was hypothesized that the intestinal microbiota composition differs between mouse models of AD and healthy controls. Therefore, the populations of four groups of bacteria, including Enterobacter, Clostridium, Lactobacillus, and Bifidobacterium species, were assessed by qPCR assays in a mouse model of AD.
Impairment in the brain glucose uptake/metabolism is a common early abnormality in AD that may be either a causative or mechanistically involved factor (17). In this study, we administrated ICV streptozotocin (ICV STZ) to mice and found that it intensified memory deficits and several AD-related brain abnormalities in AD mice. Intracerebroventricular STZ administration also caused neuroinflammation, learning and spatial memory deficits, tau hyperphosphorylation in the brain, and altered synaptic protein and insulin signaling (18). As expected, significant impairments were observed in the short-term memory and spatial reference memory of AD mice in the MWM and passive avoidance response tests. These findings are consistent with previous literature reports (19).
The results of the present study demonstrate significant differences in the fecal microbiota, including all bacterial groups assessed, between the AD mice and healthy controls. These findings are consistent with a previous study by Kowalski et al., which reported significant differences in the populations of Bifidobacterium, Lactobacillus, Clostridium, Prevotella, and Bacteroides genera, as well as Actinobacteria, Bacteroidetes, and Firmicutes phyla, between AD patients and control individuals (19). However, unlike the study by Brandscheid et al., our findings show that the populations of Lactobacillus, Clostridium, and Enterobacter were markedly higher in healthy mice relative to the AD mice (20). In another study by Cao et al. (21), higher levels of Bifidobacterium species were observed in the AD group relative to the control group. However, our study found that the population of Bifidobacterium was higher in healthy mice than in AD mice (21). This observation aligns with the findings of Kobayashi et al., who declared reduced numbers of anti-inflammatory bacteria, particularly Bifidobacterium species, and increased quantities of pro-inflammatory bacteria, such as Firmicutes and Bacteroidetes, in AD gut microflora (22). Such changes can lead to elevated inflammation levels in the plasma and CNS. Interestingly, Wang et al. in 2020, found no significant difference in the population of Bifidobacterium longum between AD and non-AD individuals in their study (23).
The current study identified significant shifts in the abundances of Enterobacter, Clostridium, Lactobacillus, and Bifidobacterium in the mouse model of AD. While the precise mechanistic pathways driving these microbiome changes remain to be fully elucidated, several potential underlying processes can be proposed. The neuroinflammation and oxidative stress characteristic of Alzheimer's pathology may directly impact the gut environment, favoring the proliferation of certain bacterial taxa like Enterobacter while suppressing others such as Bifidobacterium (24, 25). Additionally, the disruption of intestinal barrier function and increased gut permeability observed in Alzheimer's could allow greater translocation of microbial products and antigens, further shaping the composition of the microbiome (26, 27). Alterations in neurotransmitter signaling pathways, including changes in levels of acetylcholine, serotonin, and gamma-aminobutyric acid, may also selectively modulate the growth of specific bacterial populations (28). A more comprehensive investigation of these potential mechanistic links between AD pathology and gut microbiome dynamics would greatly strengthen the interpretation of the current findings.
Over the past decade, a growing number of studies have focused on using probiotics to enhance CNS function. Although most of these studies were conducted on animals, they suggest that probiotics- live microorganisms that confer health benefits when taken in sufficient amounts as per Food and Agriculture Organization (FAO) and the World Health Organization (WHO) definitions could be helpful in improving human health (29, 30). Early studies from the 1970s confirmed that specific Lactobacillus and Bifidobacterium strains were beneficial to humans (14, 31). Researchers have used various strains of probiotics and examined various CNS functions and/or dysfunctions, with consistent positive effects observed across all previous animal and human studies (32, 33).
Bifidobacterium preparations were used in most of the available studies, which proved effective in enhancing specific CNS functions (34, 35). Our study found that probiotic bacteria, such as Bifidobacterium and Lactobacillus, known for their health benefits in the gut microbiome, markedly reduced in the AD group relative to the healthy control group. This suggests that AD might have a direct correlation with decreased levels of these beneficial bacteria.
5.1. Limitations
It is important to acknowledge several key limitations of the present study. First, this investigation was conducted entirely in a mouse model of AD, which inherently restricts the generalizability of the findings to human populations. Replicating these analyses in human subjects will be an important next step to validate the applicability of the results. Additionally, the study focused specifically on four bacterial genera-Enterobacter, Clostridium, Lactobacillus, and Bifidobacterium. However, the gut microbiome is an enormously complex and diverse community, and examining changes in a broader array of microbial taxa may reveal additional important insights. Furthermore, the study did not account for potential confounding environmental factors and dietary influences that can significantly impact the gut microbiome composition. Finally, the relatively short duration of the study means that long-term longitudinal shifts in the microbiome throughout AD progression were not assessed. Acknowledging these limitations can help provide a more balanced and nuanced interpretation of the current findings and guide future research in this area.
While a growing body of evidence has highlighted the potential importance of the gut-brain axis in the context of AD (25, 27), the current investigation offers several novel and significant contributions. Rather than taking a broad, exploratory approach to characterizing microbiome changes, this study purposefully targeted four bacterial genera-Enterobacter, Clostridium, Lactobacillus, and Bifidobacterium - that have been previously implicated in Alzheimer's pathology and neuroinflammation (24, 26). By employing a targeted analytical strategy, the study was able to provide a more nuanced and mechanistically grounded examination of how specific components of the gut microbiome are altered in the context of AD progression in a mouse model. Moreover, the integration of these microbiome findings with assessments of cognitive function, amyloid-beta deposition, and neuroinflammation offers a more holistic perspective on the complex interplay between the gut and the brain in this neurodegenerative disorder. Collectively, these novel aspects of the research design and analytical approach meaningfully expand upon prior work in this domain and shed new light on the role of the gut microbiome in AD pathogenesis.
5.2. Conclusions
The findings from this comprehensive investigation of the gut microbiome in an AD mouse model underscore the potential clinical relevance of targeting specific bacterial taxa as part of a broader therapeutic strategy for this devastating neurodegenerative disorder. By demonstrating that alterations in the abundance of Enterobacter, Clostridium, Lactobacillus, and Bifidobacterium are closely linked to cognitive deficits, neuroinflammation, and amyloid-beta pathology, this study provides important mechanistic insight into how microbial dysbiosis may contribute to AD progression. Importantly, these results suggest that modulating the levels of these specific bacterial genera through dietary, probiotic, or other microbiome-targeted interventions could represent a promising avenue for future therapeutic exploration. Furthermore, the strong correlations observed between the gut microbiome, CNS outcomes, and behavioral phenotypes highlight the value of continued research into the bidirectional gut-brain axis in AD. Expanding these investigations to longitudinal human studies and clinical trials will be essential for translating these foundational findings into effective clinical applications. Collectively, this work represents an important step forward in elucidating the complex interplay between the gut microbiome and AD pathogenesis, with significant implications for the development of innovative, microbiome-based therapeutic strategies.